Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Geoacta
On-line version ISSN 1852-7744
Geoacta vol.34 no.1 Ciudad Autónoma de Buenos Aires Jan./June 2009
Sorption of fluoride by quartz sand: batch tests
Eduardo Usunoff1, Pablo Winzettel2, and Sebastián Dietrich3
1 CIC-UNCPBA, Rep. de Italia 780, Azul,
2 CIC-UNCPBA, Rep. de Italia 780, Azul, paw@faa.unicen.edu.ar
3Becario CONICET, Rep. de Italia 780, Azul, sebadietrich@faa.unicen.edu.ar
ABSTRACT
Despite the many efforts of scientists, in particular those from the field of soil science, the fate and distribution of fluorine (F) species in soils and aquifers remain relatively unraveled. As for groundwater systems, such a shortcoming makes difficult the finding and development of safe water supplies. Likewise, the use of transport models does not render acceptable results because of the many uncertainties related to the behavior of F in aqueous media. This paper presents the results of four batch test in which solutions of different pH and [F-] (concentration of fluoride) were in contact during 48 hours with clean quartz sand grains. The resulting data were fitted by linear versions of the Freundlich, the Langmuir, and the Langmuir-Freundlich models. The [F-] was varied between 0,5 and 10 mg L-1, except in one batch where a large initial concentration of F was used (45 mg L-1), and the range of pH used was 2,95 to 5,02. From a sieve analysis, the quartz grains had a medium size (d50) of 0,25 mm, and a uniformity coefficient (d40/d90) of 1,65. According to the fits and some dedicated goodness of fit indices, the Langmuir-Freundlich approach gave the best results for the batch test at the lowest pH, whereas the three remaining tests data were fitted by the Freundlich equation. It has to be mentioned that the pH of the equilibrium solutions were higher than the pH of the initial solutions, which was interpreted as an exchange process of OH- by F- on the quartz sand surface. However, such an exchange does not stand out as the exclusive mechanism promoting the F- disappearance from solution. It is deemed that the obtained results can be used as initial estimates of parameters in models used for calibrating the transport of F- in aquifers.
Keywords: F- sorption; Isotherms; Batch tests
RESUMEN
A pesar de los muchos esfuerzos de los científicos, en particular de aquellos dedicados a las ciencias del suelo, el destino y la distribución de las especies de F (flúor) en suelos y acuíferos continúan siendo aspectos no mayormente dilucidados. Con referencia a los sistemas hidrológicos, tales indefiniciones plantean dificultades con respecto a la identificación y desarrollo de fuentes seguras de agua para abastecimiento. De igual modo, los modelos de transporte no brindan resultados aceptables por las incertidumbres relacionadas con el comportamiento del F en medios acuosos saturados. Este trabajo presenta los resultados de cuatro ensayos en lote en los que soluciones de diferente pH y [F-] (concentración de fluoruro) permanecieron en contacto durante 48 horas con granos limpios de arena cuarzosa. Los datos resultantes se analizaron con referencia a versiones lineales de los modelos de Freundlich, Langmuir y Langmuir-Freundlich. La [F-] varió entre 0,5 y 10 mg L-1, excepto en un ensayo en que se utilizó una concentración de F- de 45 mg L-1, y el pH se varió en el rango de 2,95 a 5,02. Los granos de arena cuarzosa poseen un tamaño medio (d50) de 0,25 mm, y un coeficiente de uniformidad (d40/d90) de 1,65. De acuerdo con los ajustes y el uso de índices de bondad de los ajustes, el modelo de Langmuir-Freundlich fue seleccionado para el ensayo a valores de pH más bajo, en tanto que la ecuación de Freundlich ajustó los tres ensayos restantes. Debe mencionarse que el pH de las soluciones de equilibrio resultó mayor que el pH de las soluciones iniciales, y que tal característica se interpretó como un proceso de intercambio de OH- por F- en la superficie de los granos de arena cuarzosa. Sin embargo, tal intercambio no es el único mecanismo que produce la desaparición de F- de las soluciones. Se considera que los resultados obtenidos pueden utilizarse como estimaciones iniciales de los parámetros en modelos dedicados a la calibración del transporte de F- en acuíferos.
Palabras clave: Adsorción de F-; Isotermas; Ensayos en lote
INTRODUCTION
The study of the behavior of aqueous fluorine (F) species has long concerned hydrologists due to the potential health hazard associated with their concentrations, even in the range of few milligrams per liter. Even though F is only a minor constituent in most natural waters, concentrations of its natural species (mainly fluoride, F-) commonly impose severe constraints in the development and availability of safe water supplies (Murthy and Murthy, 1977; Natarajan and Mohan Rao, 1977; Ramamohana Rao et al., 1977; Rao, 1977; Gómez Artola et al., 1983; Rao, 2003).
Inasmuch as groundwater modeling techniques have proved to be among the most effective tools for exploring the fate of dissolved species, most of the efforts have focused on the assessment of parameters that control the movement of F- in aquifers. One of such transport-controlling phenomena is sorption, which, aside from expensive and cumbersome field experiences, may use the laboratory experimental approach known as batch tests. This paper deals precisely with those tests, and aims at revealing the effect of putting in contact quartz sand with solutions of varying [F-] and pH, specifically whether the F- ions are able to interact with other species on the surface of quartz sand grains.
BACKGROUND ON MATERIALS AND METHODS
Carrying out a batch test is quite straightforward: solutions of known [F-] and pH are prepared and brought into contact with a known mass of the quartz sand. Fresh F- solutions were prepared by dissolving proper amounts of NaF and their pH adjusted by using diluted solutions of HNO3. Such a slurry mix is stirred at a constant temperature and, after some time (48 hours, in this case), the equilibrium [F-] and pH are measured. The mass of F- that has disappeared from the equilibrium solution is assumed to be attached to the sand grains (i.e., adsorbed). Later on, the data are handled in such a way that the distribution or partition coefficient, Kd (Freeze and Cherry, 1979), is obtained as described below. It should be pointed out that, for analyzing batch test data, a proper quartz to water ratio (rqw) has to be used. Based on initial trials, a rqw of 0,015 kg L-1 was used in four batch experiments, numbered 1 to 4. Plastic centrifuge tubes of 50-ml capacity were use as the reaction containers, in which 0,375 g of sand and 25 ml of F- carrying solutions were poured. To eliminate impurities, the quartz sand samples, commercially known as bond sand, was boiled in 10% HCl for ten minutes and then washed with excess distilled water until the pH of the leachate equalled that of the distilled water. The samples were then oven-dried at 105 ºC and kept in close containers. The F-concentrations were measured with a Fisher Accumet pH/mV (model 620) meter and a specific electrode (Orion, model 94-09). Before measuring a given set of samples, fresh solutions of known [F-] were prepared to obtain the calibration curve, and a recheck of such a curve was done between samples measurements, following the guidelines given by Orion Research Inc. (1973) and by the U.S. Environmental Protection Agency (1979). Both samples and standards were mixed in proper amounts with TISAB, a commercially available solution designed to partially buffer the pH between 5 and 6, and to provide a high total ion strength background that swaps out variations in total ion strength between samples (Orion Research Inc., 1973). For the pH measurements, the pH meter mentioned above was used with a standard glass-combination electrode, which was calibrated against commercial buffer solutions of known pH. All [F-] and pH readings were taken at 23 ± 1 ºC.
The pH of the solutions was measured before pouring the sand, and its value labeled as initial pH (when needed, diluted nitric acid was used to adjust the pH of the batch solutions). All batch solutions contained F- in concentrations comparable to those found in natural water (0,5 to 10 mg L-1), except for batch 1 in which larger concentrations were use, up to about 45 mg L-1. Such F- solutions were labeled as initial [F-]. The batch solutions were shaken for 48 hours in a continuous-rotation shaker (although previous experiments has shown that no noticeable changes in the [F-] in solution were measured after 14-16 hours), after which the pH of the supernatant was measured following centrifugation and separation of the solid phase. After adding TISAB, the F- content of the supernatant was measured, and the readings labeled as final [F-]. The amount of F- absorbed was obtained by subtracting the final [F-] from the initial [F-]. The [F-] readings were normalized considering the rqw.
Processing of the batch tests data made use of the so-called adsorption isotherms (the isotherm word is used because the experiments take place at constant temperature). Although such isotherms may have at least four different shapes (Morrill et al., 1982), the results are normally described by the Langmuir equation, the Freundlich equation, or a hybrid combination of both.
The Langmuir equation
It was originally developed for adsorption of gases on solids (Langmuir, 1918), although is long ago used for describing adsorption of species from solution. It is expressed as follows:
where S is the adsorbed concentration (mg kg-1), Smax is the maximum sorption capacity of the solid phase (mg kg-1), K is the Langmuir binding-strength coefficient (L mg-1), and C is the equilibrium concentration (mg L-1).
Notice that the Langmuir model is nonlinear; hence, fitting this model to measured data requires a trial and error approach. Furthermore, S is not measured but estimated from the initial and final concentrations of the solute, assuming that adsorption is the exclusive mechanism that takes place in the system.
The Freundlich equation
An alternative model for evaluating batch tests data is the Freundlich equation (Freeze and Cherry, 1979), which can be written as follows:
where S and C are as above, Kd (L kg-1) is the equilibrium constant (also known as partition coefficient or distribution coefficient), and n is a constant usually less than one and indicative of the degree of linearity of the adsorption process. Sposito (1984) stresses that the Freundlich isotherm only applies to data obtained at low values of C. Again, the Freundlich model is not linear, unless n ≈ 1. As said before, S is not measured but estimated from solute initial and final concentrations.
The Langmuir-Freundlich equation
Sposito (1980) developed a power function, otherwise known as the exponential Langmuir model, which reads as follows:
S = Smax K Cß / 1 + K Cß (3)
where S, Smax, K, and C are as before, and ß is a fitting parameter (0 < ß < 1).
The Bolster-Hornberger approach
Due to the limitations of the models above, chiefly their non-linear nature, many authors have investigated the possibility on using linearized version of equations 1, 2, and 3. One successfully tested method has recently been proposed by Bolster and Hornberger (2007).
They came up with a Microsoft Excel© spreadsheet, available at http://ars.usda.gov/msa/awmru/bolster/Sorption_spreadsheets, which uses the method of least squares regression for fitting the nonlinear equations to the measured adsorption data. That is achieved by minimazing the sum of the squared errors (SSE) between observed and calculated values, also known as the residual sum of squares. For the adsorbed concentration (S), the method minimizes the following objective function:
SSE = Σ wi [Si - Si]2(i=1,.,N) (4)
where N is the number of observations, wi is the ith weighting factor (weighted: w=1/S2; unweighted: w=1), Si is the ith measured value of the dependent variable (S, in this case), and Si is the ith model-predicted value of the dependent variable.
When comparing different models, however, it is not a good idea to select the one that has the lowest SSE (if the models have different number of parameters, the minimization of SSE will always render lower values for the model that has more parameters). Another goodness of fit measure is given by the corrected Akaike's Information Criterion or AIC (Burnham and Anderson, 2002). In essence, and as long as the number of data points exceed the number of fitting parameters by five or more, the model with the lowest AIC is considered to be the most likely to be correct.
Finally, Bolster and Hornberger (2007) considered a third method for determining the goodness of fit: the model efficiency, E (Nash and Sutcliffe, 1970). E is calculated as:
E = 1 - [{ Σ wi [Si - Si]2}/ { Σ wi [Si - Swavg]2}}(i=1,.,N) (5)
where all symbols have been defined, but Swavg, which is the weighted mean of the measured values. It can easily be appreciated that an E equal to 1 means a perfect fit to the data, whereas values < 0 indicates that the model prediction is not better than the average of all the measured values.
RESULTS
The results obtained in the batch test are displayed in Table 1. It should be noticed that the estimated S values have been already normalized taking into account the rqw. Such results highlight two facts: (a) there is an effective uptake of F- from the batch solutions, and (b) there is an increase in pH in the equilibrium solutions with respect to the initial solutions (such increase is not registered in batch 1). It would appear that the change in pH increases as the pH of the initial solutions is higher.
Table 1. Measured data of initial [F-], equilibrium [F-], initial and final pH.

Given the linearization approach developed by Bolster and Hornberger (2007), the batch tests data considered the Freundlich, Langmuir, and Langmuir-Freundlich models, which yielded the parameters and statistics shown in Table 2.
Table 2. Fitted parameters and goodness of fit statistics.

Shaded cells denote the best model according to the goodness of fit indices
In view of the results of Table 2, the "best" models were selected and the measured data and fitted functions were plotted in Figures 1, 2, 3, and 4.

Figure 1. Batch 1: Langmuir-Freundlich model fit. (initial pH»2,95; initial [F] range: 0,99-45,30 mg L"1)

Figure 2. Batch 2: Freundlich model fit. (initial pH»4,23; initial [F] range: 0,99-10,70 mg L"1)

Figure 3. Batch 3: Freundlich model fit. (initial pH»4,41; initial [F] range: 0,99-10,70 mg L"1)
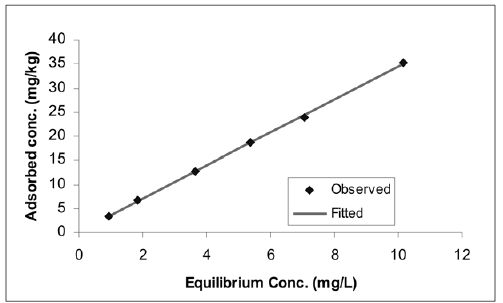
Figure 4. Batch 4: Freundlich model fit. (initial pH»5,02; initial [F] range: 0,99-10,70 mg L"1)
As mentioned above, the increase in pH in the equilibrium solutions as compared to the start-up solutions may be attributed to an exchange (release) of OH- for F- on the surface of the quartz sand grains. If so, there should be some kind of relationship between the [OH-] and the adsorbed [F-], which may be discussed taking into account Figure 5.

Figure 5. F- adsorbed by quartz sand and OH- released to equilibrium solutions (data of batch 1 virtually coincide with the X-axis)
DISCUSSION
Although the parameters from the different models considered differ, the goodness of fit statistics are not very far apart (Table 2). Fortunately, such goodness of fit indices helped pointing the "best" models based on the lowest SSE and AIC, and the E close to unity. It has to be mentioned that the linearization proposed by Bolster and Hornberger (2007) was carried out considering unweighted measurements (w=1 in equation 4). The fact is, by virtue of the measurement procedure, that there are no reasons to believe that a given reading is better than the others. That does not mean that the possibility or errors is disregarded; it only assumes that if measurement errors exist, they have equal effects in all batch tests.
As for the eventual replacement of OH- by F- on the quartz sand grains surface, the process does not entirely justify the uptake of F- from the batch solutions. If it were so, the observed F- adsorbed data would lie very close to the stoichiometric replacement line (Figure 5). Fan et al. (2003) reported a replacement of F- ions for OH- groups on quartz surfaces, although the rqw they considered (16,7) is much higher that the one used in the experiments described in this paper (0,015). It would seem that such a direct anion exchange is more justified at low pH, a finding reported in U.S. Environmental Protection Agency (1999). Another supporting argument is given by Perrott et al. (1976), who found that a sample of quartz from Brazil released 1 mmol of OH- per 100 g of sample after 25 minutes in contact with a solution of NaF 0,85 M. Of course, it has to be known the solid phase zero-point-of-charge, pHzpc, which is the pH where the surface has a zero net charge. Incidentally, the pHzpc for SiO2 (silica) is 2, according to Stumm and Morgan (1981). That would explain the rather meager change in pH of batch 1 (Table 1) and the slight adsorption of F- related to OH- release.
A final comment should be made about the fluorine complexation at low pH and the eventual effect of F on the dissolution of quartz. At pH below neutrality and in the absence of cations such as Al3+ and Fe3+, the F species able to be present are HF0, HF-2, and F- (Hem, 1968; Roberson and Barnes, 1978). The concentrations of HF0 and HF-2 (at 25 °C) can be calculated from (Broene and De Vries, 1947):
[HF0] = [H+] [F-] 103,17, and [HF-2] = [H+] [F-]2 103,76
Hence, unless the pH is extremely low and the total [F-] is very high, the amount of HF-2 in solution can be neglected. It is of particular importance the [HF0] in batch 1. A simple calculation demonstrates that most of the F is in the HF0 form. However, as said before, TISAB is added before measuring the F content, so that the pH is buffered between 5 and 6 and the variations of the ion strength among samples can be ignored. Therefore, all readings correspond to [F-].
As for the possible species that F can form with silicon, Roberson and Barnes (1978) concluded that SiF-6 is the most important one. However, the solubility of quartz at 25 °C is so low (6 mg L-1) (Morey et al., 1962; Marion et al., 1976; Roberson and Barnes, 1978) that, even for the batch test at the lowest pH (batch 1), the [SiF-6] barely exceeds 10-8 M, and it can safely be disregarded. Harouiya and Oelkers (2004) have reported that the presence of 1x10-3 mol kg-1 of NaF increases the quartz dissolution rate by a factor between 2,5 and 3 with respect to NaF-free solutions. Such findings come from experiments carried out at a pH of 2 and temperatures of 50 and 100 °C, which are hardly comparable to the respective values of pH and temperatures of the batch test here presented.
CONCLUSIONS
The batch experiments, which brought into contact solutions of varying [F-] and pH with clean grains of quartz sand until equilibrium conditions were achieved, revealed that quartz (a rather non reactive mineral at the experimental pH) is able to adsorb minute amounts of F- ions. It is not clear the adsorption mechanisms, although it would partially be attributed to anion exchange between OH- and F- onto the mineral surface, particularly at low pH.
The models used to analyze the data yielded similar results in terms of observed measurements versus fitted values, although the parameters from the fitting exercise vary widely. The only way for choosing the "best" model was the linearization of the models and the consideration of the goodness of fit statistics. The models so chosen were the Langmuir-Freundlich for the batch test at the lowest pH, and the Freundlich equation for the rest.
In spite of the many drawbacks that batch tests may have, they are able to provide initial estimates of parameters which are relevant for solute transport studies. It is important to note that scientists knowledgeable of sorption processes in natural environments have long known that generic or default partition coefficient values found in the literature can result in significant errors when used to predict the absolute impacts of contaminant migration or site-remediation options.
REFERENCES
1. Bolster, C. and G. Hornberger. 2007. "On the use of linearized Langmuir equations". Soil Science Society of America Journal 71, pp. 1796-1806. [ Links ]
2. Broene, H. and T. De Vries. 1947. "The thermodynamics of aqueous hydrofluoric acid". Journal of the American Chemical Society 69, pp. 1644-1646. [ Links ]
3. Burnham, K. and D. Anderson. 2002. Model selection and multimodel inference: A practical information-theoretic approach. Springer. [ Links ]
4. Fan, X., D. Parker, and M. Smith. 2003. "Adsorption kinetics of fluoride on low cost materials". Water Research 13, pp. 4929-4937. [ Links ]
5. Freeze, R. and J. Cherry. 1979. Groundwater. Prentice-Hall, 604 p. [ Links ]
6. Gómez Artola, C., J. Borregón Martínez, M. Llamas Madurga y J. Sánchez Sáez. 1983. "Análisis preliminar del contenido de flúor de las aguas de Madrid y de su incidencia en las caries dentales de los niños". Actas del III Simposio Nacional de Hidrogeología, pp. 157-166. [ Links ]
7. Harouiya, N, and E. Oelkers. 2004. "An experimental study of the effect of aqueous fluoride on quartz and alkali-feldspar dissolution rates". Chemical Geology 205, pp. 155-167. [ Links ]
8. Hem, J. 1968. "Graphical methods for studies of aqueous aluminum hydroxide, fluoride, and sulfate complexes". Geol. Survey Water-Supply Paper 1827-B, 33 p. [ Links ]
9. Langmuir, I. 1918. "The adsorption of gases on plane surfaces of glass, mica, and platinum". Journal of the American Chemical Society 40, pp. 1361-1403. [ Links ]
10. Marion, G., D. Hendricks, G. Dutt, and W. Fuller. 1976. "Aluminum and silica solubility in soils". Soil Science 121, N° 2, pp. 76-84. [ Links ]
11. Morey, G., R. Fournier, and J. Rowe. 1962. "The solubility of quartz in the temperature interval from 25 °C to 300 °C". Geochimica et Cosmochimica Acta 26, pp. 1029-1043. [ Links ]
12. Morrill, L., B. Mahilum, and S. Mohiuddin. 1982. Organic compounds in soils: Sorption, degradation, and persistence. Ann Arbor Science, 326 p. [ Links ]
13. Murthy, D.S. and D.V. Murthy. 1977. "A study of high fluoride bearing waters around Nalgonda District, Andhra Pradesh". Proceedings of the 1974 Symposium on Fluorosis, Indian Academy of Geosciences, pp. 311-315. [ Links ]
14. Nash, J. and J Sutcliffe. 1970. "River flow forecasting through conceptual models, Part 1: A discussion of principles". Journal of Hydrology 10, pp. 282-290. [ Links ]
15. Natarajan, V. and V.R. Mohan Rao. 1977. "Hydrochemical investigation for fluorine-bearing minerals in the Kangall and Hallia River Basins, Nalgonda District, Andhra Pradesh". Proceedings of the 1974 Symposium on Fluorosis, Indian Academy of Geosciences, pp. 37-47. [ Links ]
16. Orion Research, Inc. 1973. Instruction manual. Fluoride electrodes models 96-09 and 94-09. Orion Res., Inc., 28 p. [ Links ]
17. Perrott, K. B. Smith, and R. Inkson. 1976. "The reaction of fluoride with soils and soil minerals". Journal of Soil Science 27, pp. 58-67. [ Links ]
18. Ramamohana Rao, N.V., K. Rajyalakshami, K.R. Krishna Sastry, P. Subba Rao, V.V. Krishna Rao, and K. Narasimha Rao. 1977. "Incidence of fluorosis in drinking water resources in Andhra Pradesh". Proceedings of the 1974 Symposium on Fluorosis, Indian Academy of Geosciences, pp. 227-235. [ Links ]
19. Rao, A.S. 1977. "Hydrogeological studies in parts of Nandigama Taluk, Krishna District, Andhra Pradesh, with special reference to the problem of fluorosis". Proceedings of the 1974 Symposium on Fluorosis, Indian Academy of Geosciences, pp. 206-216. [ Links ]
20. Rao, N. 2003. "Fluoride and environment- A Review". Proceedings of the Third International Conference on Environment and Health, University of Madras, pp. 386-399. [ Links ]
21. Roberson, C. and R. Barnes. 1978. "Stability of fluoride complex with silica and its distribution in natural water systems". Chemical Geology 21, pp. 239-256. [ Links ]
22. Sposito, G. 1980. "Derivation of the Freundlich equation for ion exchange reactions in soils". Soil Science Society of America Journal 44, pp. 652-654. [ Links ]
23. Sposito, G. 1984. The Surface Chemistry of Soils. Oxford University Press, New York, New York. [ Links ]
24. Stumm, W. and J. J. Morgan. 1981. Aquatic Chemistry. An Introduction Emphasizing Chemical Equilibria in Natural Waters. John Wiley and Sons, New York, New York. [ Links ]
25. U.S. Environmental Protection Agency. 1979. Methods for chemical analysis of water and wastes, 1978. Env. Mon. and Support Lab., Office Res. and Develop., 441 p. [ Links ]
26. U.S. Environmental Protection Agency. 1999. Understanding variation in partition coefficient, Kd, values. Volume I: The Kd Model, Methods of Measurement, and Application of Chemical Reaction Codes. United States Office of Air and Radiation EPA 402-R-99-004A, 212 p. [ Links ]














