Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista del Museo Argentino de Ciencias Naturales
versión On-line ISSN 1853-0400
Rev. Mus. Argent. Cienc. Nat. vol.19 no.1 Ciudad Autónoma de Buenos Aires jun. 2017
BOTÁNICA
Pollen morphology of Proteaceae native to Argentina: a new dichotomus key for their identification
Damián Andrés Fernández
Sección Paleopalinología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. A. Gallardo 470, C1405DJR Buenos Aires, Argentina. E-mail: dafernandez@macn.gov.ar.
Abstract
A dichotomous key for the identification of eight proteaceous species, native to Argentina, based on pollen morphology is presented. These species are: Embothrium coccineum, Gevuina avellana, Lomatia dentata, L. ferruginea, L. hirsuta, Orites myrtoidea, Roupala meisneri and R. montana. The pollen morphology was analyzed with both, light and electron scanning microscopes. The morphological characters selected for species/genera recognition are: ornamentation, pore number, equatorial diameter, the ratio of sexine/nexine thickness, and equatorial diameter/pore diameter ratio.
Key words: Proteaceae; Argentina; Palynology; Dichotomous key.
Resumen
Morfología polínica de las Proteaceae nativas de Argentina: una nueva clave dicotómica para su identificación
Se presenta una clave dicotómica para la identificación de las ocho especies de Proteacea nativas de la Argentina. Las especies son: Embothrium coccineum, Gevuina avellana, Lomatia dentata, L. ferruginea, L. hirsuta, Orites myrtoidea, Roupala meisneri and R. montana. La morfología polínica fue analizada utilizando microscopio óptico y microscopio electrónico de barrido. Los caracteres morfológicos elegidos para reconocer las especies/géneros son: tipo de ornamentación, número de poros, diámetro ecuatorial, relación de espesor sexina/nexina y relación diámetro ecuatorial/diámetro de poro.
Palabras clave: Proteaceae; Argentina; Palinología; Clave dicotómica.
INTRODUCTION
The Proteaceae family comprises 83 genera and about 1660 species of trees or shrubs (Christenhusz & Byng, 2016), which are predominantly distributed in the Southern Hemisphere. The Proteaceae are considered a relictual clade from Gondwana because of its disjunct modern distribution in Australia, Africa, Central and South America, and in limited places in Asia and the Pacific Islands (Johnson & Briggs, 1963, 1975, 1981; Weston & Crisp, 1994). The family has a rich fossil record dating back to the Late Cretaceous. Macro and micro fossils are known from across the Southern Hemisphere, including Antarctica. For this reason, Proteaceae is a key family for understanding the biogeography of this region (Askin & Baldoni, 1998; González, 2007; Carpenter, 2012).
The accepted phylogenetic position of Proteaceae is sister to Platanaceae (APGIII, 2009). According to Weston & Barker (2006), three tribes within Grevilleoideae (Embothrieae, Macadamieae, and Oriteae) are currently found in Argentina. The Embothrieae are represented by two genera, the monospecific Embothrium Forst., endemic to South America, and Lomatia R.Br., which is found in Australia and South America. The Macadamieae are represented by two genera, the South American endemic Gevuina Molina and the South and Central American endemic Roupala Aubl.. Oriteae contains the genus Orites R.Br., which is restricted to temperate regions of Australia and southern South America (Prance & Plana, 1998).
The American species of Proteaceae are distributed in two major groups based on how and when they dispersed to South America (Johnson and Briggs, 1975). The first group inhabits the Patagonian subantarctic forests and high altitudes in the Andes and includes the genera Lomatia, Embothrium, Gevuina and Orites. The Argentinean species of these genera are: Embothrium coccineum J.R. Forst. & G. Forst., Gevuina avellana Molina, Lomatia dentata (Ruiz & Pav.) R. Br., L. ferruginea (Cav.) R. Br., L. hirsuta (Lam.) Diels, Orites myrtoidea (Poepp. & Endl.) Benth. & Hook. F. ex B.D. Jacks (Fig. 1, in black). Representatives of the second group occur in subtropical and tropical forests from central and northern South America and Central America. The latter group includes Panopsis, Euplassa and Roupala (Prance and Plana, 1998). In Argentina only Roupala meisneri Sleumer and Roupala montana Aubl. var. brasiliensis (Klotzsch) K.S. Edwards grow in Yungas and Paranense rainforest (Fig. 1, in grey). According to Johnson and Briggs (1975) the southern group would have reached Patagonia via Antarctica during the early Paleocene and the northern group was supposed to arrive from Africa before the breakup of Gondwana in the Turonian. These groups do not reflect systematic relationships corroborating radiation of the family prior to the separation of these continents (Pujana, 2007).
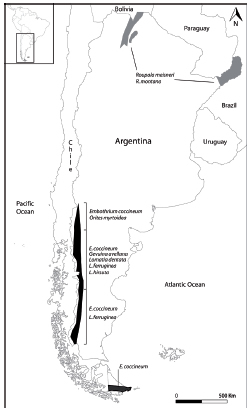
Fig. 1. Distribution area of Proteaceae in Argentina. Bosque Andino Patagónico species in dark grey; Roupala meisneri and R. montana var. brasiliensis in light grey (Cabrera 1976, Belgrano et al. 2008).
The broad pollen morphology for the Patagonian Proteaceae was described by Hebel & Rojas (2000) from Chilean specimens. There is one report on the general pollen morphology for the Brazilian Roupala species but it does not provide photomicrographs of the specimens studied (Barth & Barbosa, 1971).
The aim of this paper is to describe in detail the pollen morphology of the eight extant Proteaceae native to Argentina using light microscopy (LM) and scanning electron microscopy (SEM) and present a key for their identification. This information would be very useful for identifying proteaceous taxa in the fossil record as well.
MATERIAL AND METHODS
Pollen grains were removed from anthers of herbarium specimens from BA, CONC and SI (Holmgrem et al., 1981). Pollen terminology follows Punt et al. (2007).
For LM pollen was acetolysed according to Erdtman (1960) and slides were prepared by mounting the pollen in glycerol jelly and sealed with paraffin. For SEM, acetolysed and non-acetolysed pollen grains were suspended in 90 % ethanol, mounted on stubs and examined using a Philips XL30 TMP SEM at the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”. The dichotomous key presented is based only on LM to make its use simpler in extant and fossil material. The grains were studied using SEM to observe their sculpture in detail.
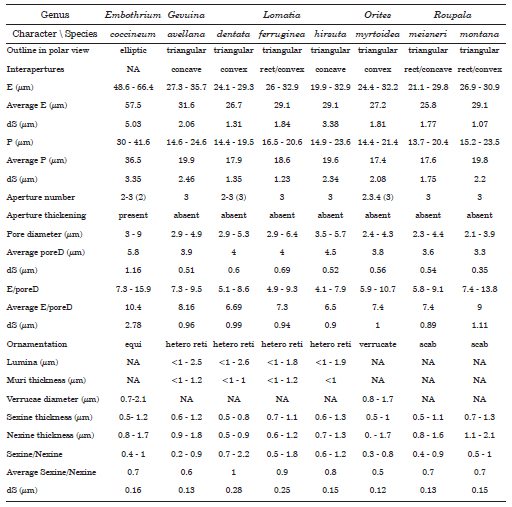
Equatorial diameter, polar diameter, pore diameter, lumina and muri dimensions, and sexine and nexine thickness were measured on 30 pollen grains under LM (Table 1). The ratio of average equatorial diameter/pore diameter (AverageE/poreD) is used as a measure of the aperture size in relation to the grain size (i.e., the bigger the ratio, the samaller the pores in relation to de grain size). For all other features at least 30 measurements were made, often with multiple measurements taken from one pollen grain or section. The statistical data for each sample was calculated using Microsoft Excel 2013.
Table 1. Pollen morphological data. Abbreviations used: E=equatorial diameter, dS=standard deviation, P=polar diameter, poreD=pore diameter, equi= equinate, hetero reti= heterobrochate reticulum, scab= scabrate.
Studied specimens
Embothrium coccineum: Hafsik (BA 83833). Gevuina avellana: M. Buchinger-Lourteig (BA 83860). Lomatia dentata: Ángel L. Cabrera (BA 19350). Lomatia ferruginea: Perez Moreau (BA 69046). Lomatia hirsuta: Kühnemann (BA 37257). Orites myrtoidea: NN (CONC 8773). Roupala meisneri: Sehnem (SI 3141). Roupala montana var. brasiliensis: Rodriguez (BA 41473).
GENERAL POLLEN CHARACTERS OF THE PROTEACEAE FAMILY
Pollen grains are isopolar (Figs. 2-3, 5-10) or anisopolar (Fig. 4); radiosymetric (Figs. 2-3, 5-10) or with bilateral symmetry (Fig. 4); oblate triangular (Figs. 2-3, 5-10) or elliptic (Fig. 4) in polar view; mesoporia straight (Figs. 7A, 9A, 10A), concave (Figs. 7C, 10C) or convex (Figs. 4, 5A, 8A, 9C); porate, with circular to subcircular pores, equatorial (Figs. 2-3, 5-10) or subequatorially arranged (Fig. 4A, C-D); exine tectate (Figs. 2-6) or semitectate (Figs. 7-10); ornamentation reticulate (Figs. 7-10), equinate (Fig. 4), verrucate (Figs. 5-6) or scabrate (Figs. 2-3). Sexine generally thinner than nexine. At the apertural level the nexine has variable thickness depending on the presence of vestibulum, it is similar or thinner than in mesoporia when the vestibulum is small and inconspicuous (usually, cavity with granular elements) (Figs. 2A-B, 3A-B, 5, 6A-B, 7A-B, 8A-B, 9A-B, 10A-B); or, it is slightly thicker than the interapertural nexine when the vestibulum is absent (Fig. 4A-B). The analyses using SEM confirm the LM observations, except in Roupala meisneri and R. montana in which SEM reveals that the exine is granulated.

Fig. 2. Roupala meisneri. A. Pollen grain in polar view under LM. B. Equatorial view under LM. C. Subpolar view under SEM. D. Detail of the granulose ornamentation next to a pore. Scale bars for A-C: 20 μm and for D: 10 μm.
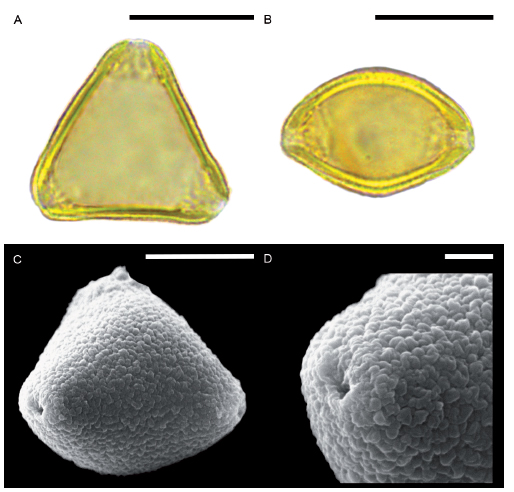
Fig. 3. Roupala montana var. brasiliensis. A. Pollen grain in polar view under LM, vestibulum conspicuous. B. Equatorial view under LM. C. Subpolar view under SEM. D. Detail of the granulose ornamentation next to a pore. Scale bars for A-C: 20 μm and for D: 2 μm.

Fig. 4. Embothrium coccineum. A. Diporate pollen grain in equatorial view under LM. B. Triporate pollen grain in polar view under LM. C. Diporate pollen grain in equatorial view under SEM. D. Triporate pollen grain in polar view under SEM. E. Detail of the ornamentation, spinae, verrucae and gemmae. F. Detail of the ornamentation, spinae curved. Scale bars for A-C and E: 20 μm; and for D-F: 5 μm.

Fig. 5. Orites myrtoidea. A. Pollen grain in polar view under LM. B. Equatorial view under LM. Scale bars for 10 μm.

Fig. 6. Orites myrtoidea. A. Pollen grain in polar view under SEM. B. Detail of verrucae next to a pore. C. Equatorial view under SEM. D. Detail of a pore. E. Tetraaperturate pollen grain in polar view under SEM. F. Detail of the polar area of E. Scale bars for A,C and E: 10 μm and for B, D, F: 2 μm.

Fig. 7. Gevuina avellana. A. Pollen grain in polar view under LM. B. Equatorial view under LM. C. Polar view under SEM. D. Detail of the reticulum, lumina decreases gradually from the equatorial half of the apoporium. E. Equatorial view under SEM. F. Detail of a pore. Scale bars for A-C and E: 10 μm; for D: 5 μm and for F: 2 μm.
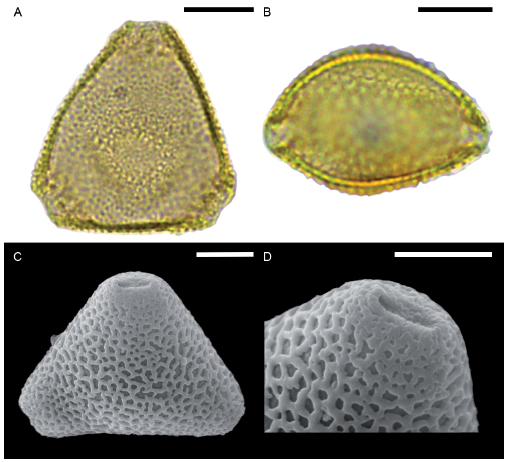
Fig. 8. Lomatia dentata. A. Pollen grain in polar view under LM. B. Equatorial view under LM. C. Subpolar view under SEM. D. Detail of the reticulum, lumina decreases abruptly close to the pore. Scale bars for A-C: 10 μm and for D: 5 μm.

Fig. 9. Lomatia ferruginea. A. Pollen grain in polar view under LM. B. Equatorial view under LM. C. Polar view under SEM. D. Detail of the reticulum, lumina decreases abruptly close to the pore. Scale bars for A-B: 20 μm; for C: 10 μm and for D: 5 μm.

Fig. 10. Lomatia hirsuta. A. Pollen grain in polar view under LM. B. Equatorial view under LM. C. Polar view under SEM. D. Detail of the reticulum, lumina decreases abruptly close to the pore. . Scale bars for A-B: 20 μm; for C: 10 μm and for D: 5 μm.
Embothrium coccineum: grains equatorial or subequatorial diporate (triporate = 2%, n = 103). Spines and verrucae uneven-sized, distinguishable in optical section (Fig. 4A-B), scattered gemmae also present. The exine is echinate and the surface is psilate between the sculpture. Spines generally conical, sometimes curved (Fig. 4C-F). Small nexine thickening at the aperture level (Fig. 4A-B).
Gevuina avellana: grains triporate, semitectate, reticulate heterobrochate. The reticulum turns gradually into a microreticulum from the second half of the apoporium to the pore (Fig. 7C-F).
Lomatia dentata, L. ferruginea and L. hirsuta: pollen morphology indistinguishable, overlap in all the measured features (Table 1). Reticulate heterobrochate. The reticulum turns abruptly into a microreticulum (Figs. 8D, 9D, 10D). Small vestibulum less conspicuous in L. dentata (Fig. 8A).
Orites myrtoidea: grains triporate (tetraporate = 7.93%, diporate = 0.88%, n = 227). Verrucae with irregular shape, uneven-sized, irregularly distributed on the grain surface, occasionally gemmae (Figs. 5, 6). The exine is scabrate between the verrucae (Fig. 6). The exine is thicker than the other species studied. Small vestibulum very inconspicuous (Fig. 5).
Roupala meisneri: scabrate under LM, granulose using SEM (Fig. 2). Small vestibulum less conspicuous than in R. montana var. brasiliensis (Fig. 2A-B).
Roupala montana var. brasiliensis: pores smaller than R. meisneri (average E/poreD=9) (Table 1). Scabrate under LM, granulose using SEM (Fig. 3). Vestibulum more conspicuous than in R. meisneri (Fig. 3A-B).
Palynological key of Proteaceae species native to Argentina
1. Pollen grains scabrate.
Genus Roupala (Fig. 2-3)
1´. Pollen grains equinate, verrucate or reticulate.
2. Ornamentation equinate. Generally diporate. E > 40 μm. Nexine thickening at the aperture level.
Embothrium coccineum (Fig. 4)
2´. Ornamentation verrucate or reticulate. Generally triporate. E < 40 μm. Vestibulum present.
3. Ornamentation verrucate.
Orites myrtoidea (Fig. 5-6)
3´. Ornamentation reticulate.
4. Lumina decreases gradually from the equatorial half of the apoporium. Sexine relatively thin (Average Sexine/Nexine = 0.6). Pores relatively small (Average E/poreD=8.16).
Gevuina avellana (Fig. 7)
4´. Lumina decreases abruptly close to the pores. Sexine relatively thick (Average Sexine/Nexine = 0.8 - 1). Pores relatively large (Average E/poreD=6.5 – 7.3).
Genus Lomatia (Fig. 8-10)
DISCUSSION AND CONCLUSIONS
Many of the details of pollen morphology of Argentinean Proteaceae have remained relatively poorly defined, probably because of the limitations of using LM to examine such pollen. This study provides better resolution of the sculpturing in the studies species’ pollen grains. In the case of the reticulate species the SEM analysis shows the nature of the changes in lumen size. In Embothrium coccineum, the SEM analysis allowed to distinguish the presence of gems. In the case of the species of the genus Roupala (wich had not been previously illustrated in detail), it revealed the granules of its surface. While these results are generally consistent with previous reports on pollen features in Chilean (Hebel & Rojas, 2000) and Brazilian (Barth & Barbosa, 1971) specimens, some differences have been discovered: 1) Argentinean Embothrium coccineum and Gevuina avellana shows smaller dimensions for all the features studied than Brazilian specimens; 2) Chilean Lomatia dentata, L. ferruginea and L. hirsuta present bigger pores and lumina than the specimens analyzed here; 3) Brazilian Roupala reach larger equatorial and polar diameters than Argentinean specimens.
Pollen grains of species belonging to the same genus are fairly indistinguishable in this analysis. However, some differences were found: Lomatia dentata shows a vestibulum less conspicuous than the other Argentinean species of the genus. It also presents a small equatorial diameter. This is consistent with the observations made by Hebel & Rojas (2000) of Chilean specimens. Roupala montana var. brasiliensis shows smaller pores (Average E/poreD=9) and a more conspicuous vestibulum than in R. meisneri. These observations suggest that more detailed studies, including pollen variability are needed.
It is interesting to note that the northern Argentinean species, Roupala meisneri and R. montana var. brasiliensis, present grains with a simple sculpture that is typically scabrate; while the Patagonian group seems to show more diverse sculpturing that is never scabrate. These morphological differences could support Johnson and Briggs (1975) hypothesis mentioned above, which distinguish two major groups of Proteaceae based on how and when they dispersed to South America. The tropical group is believed to have arrived from Africa in the Turonian (93–89 million years ago), while the southern group may have reached Patagonia via Antarctica during the early Paleocene (66–56 million years ago). Thus, the simpler pollen morphology of the tropical Proteaceae may be the result of a long independent evolutionary history in America. Future works should address the relationship between the pollen morphology, systematics, and paleobiogeography of the group.
This study provides the first dichotomous key to the Proteaceae native to Argentina based on their pollen morphology. The key and pollen descriptions presented will assist the palynologists attempting to identify the pollen grains of Proteaceae in Argentina. The pollen features that allow differentiation of genera and species are: ornamentation, number of apertures, equatorial diameter, ratio sexine/nexine thickness and ratio equatorial diameter/pore diameter. All of these features are easily recognizable using LM, making them good characters for use in future palynological studies. Many fossil pollen grains putatively related to the family still have an uncertain affinity, so it is crucial to enrich our understanding of extant species in this important family. This study is a much-needed addition to our understanding of extant proteaceous pollen morphology.
ACKNOWLEDGEMENTS
The author wish to express his thanks to: Lilia Mautino and an anonymous reviewer for their valuable corrections, comments and suggestions, which improved the quality of the manuscript; Fabián Tricárico for his technical assistance; the authorities of BA, CONC and SI for allowing the use of herbarium material for this study; Viviana Barreda, Luis Palazzesi, and M. Cristina Tellería for their comments on the manuscript; and, Alexander Bippus for his help improving English. Financial support was provided by Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIP 2014–0259).
BIBLIOGRAPHY
1. APG, III (The Angiosperm Phylogeny Group). 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105-121. [ Links ]
2. Askin, R.A., & A.M. Baldoni. 1998. The Santonian through Paleogene record of Proteaceae in the southern South America–Antarctic peninsula region. Australian Systematic Botany 11(4): 373-390.
3. Barth, O.M. & A.F. Barbosa. 1971. Catalogo sistematico dos polens das plantas arboreas do Brasil Meridional - Proteaceae. Memorias do Instituto Oswaldo Cruz 69(3): 425-435. [ Links ]
4. Belgrano, M.J., O. Morrone & F.O. Zuloaga. 2008. Catálogo de las plantas vasculares del Cono Sur: (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Instituto de Botánica Darwinion San Isidro, St. Louis, Mo., U.S.A.: Missouri Botanical Garden Press. [ Links ]
5. Cabrera, A.L. 1976. Regiones fitogeográficas argentinas. In: Enciclopedia Argentina de Agricultura y Jardinería I. 2da edición. Acme, Buenos Aires, pp. 85. [ Links ]
6. Carpenter, R.J. 2012. Proteaceae leaf fossils: phylogeny, diversity, ecology and austral distributions. The Botanical Review 78(3): 261-287. [ Links ]
7. Christenhusz, M.J. & J.W. Byng. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261(3): 201-217. [ Links ]
8. Erdtman G. 1960. The acetolysis method. A revised description. Sven. Bot. Tidskr., 54: 561-564. [ Links ]
9. González, C.C., M.A. Gandolfo, N.R. Cúneo, P. Wilf & K.R. Johnson. 2007. Revision of the Proteaceae macrofossil record from Patagonia, Argentina. Botanical Review 73(3): 235-266. [ Links ]
10. Hebel, I. & G. Rojas. 2000. Morfología de los granos de polen de especies de la familia Proteaceae presentes en Chile. Boletín del Museo Nacional de Historia Natural 49: 51-72. [ Links ]
11. Holmgrem P.K., W. Keuken & E.K. Schofield. 1981. Index Herbariorum, Part 1: The Herbaria of the World vol. 106. 7th edition. Bohn, Scheltema and Holkema, Utrecht, 7 pp. [ Links ]
12. Johnson, L.A.S. & B.G. Briggs. 1963. Evolution in the Proteaceae. Australian Systematic Botany 11(1): 21-61. [ Links ]
13. Johnson, L.A.S. & B.G. Briggs. 1975. On the Proteaceae - the evolution and classification of a southern family. Botanical journal of the Linnean Society 70(2): 83-182. [ Links ]
14. Johnson, L.A.S. & B.G. Briggs. 1981. Three old southern families Myrtaceae, Proteaceae and Restoniaceae. In: A. Keast (ed.), Ecological biogeography of Australia, pp. 429-469, Dr. W. Junk bv Publishers, The Hague. [ Links ]
15. Prance, G. T. & V. Plana. 1998. The American Proteaceae. Australian Systematic Botany 11(4): 287-299. [ Links ]
16. Pujana, R. R. 2007. New fossil woods of Proteaceae from the Oligocene of southern Patagonia. Australian Systematic Botany 20(2): 119-125. [ Links ]
17. Punt, W., P.P. Hoen, S. Blackmore, S. Nilsson & A. Le Thomas. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143(1): 1-81. [ Links ]
18. Weston, P.H. & M.D. Crisp. 1994. Cladistic biogeography of waratahs (Proteaceae, Embothrieae) and their allies across the pacific. Australian Systematic Botany 7(3): 225-249. [ Links ]
19. Weston, P.H. & N.P. Barker. 2006. A new suprageneric classification of the Proteaceae, with an annotated checklist of genera. Telopea 11(3): 314-344. [ Links ]
Recibido: 21-IV-2017
Aceptado: 24-V-2017














