INTRODUCTION
The number of studies on parasitic metazoan communities and estimation of the relative bio diversity of parasites to the search relationships parasite-host in marine bony fishes in Peru have increased in recent years (Céspedes-Chombo et al., 2017; Chero et al., 2019; Ferré-Alcántara et al., 2019; Londoñe-Bailón et al., 2020; Minaya et al., 2020a,b). Currently, there is a remarkable relevance in studies on the biodiversity of the parasitic fauna because they are important for maintaining the balance in the ecosystem by reg ulating the density and abundance of their hosts, structuring food chains (Poulin et al., 2016). Unfortunately, the same cannot be said for par asitological studies in elasmobranch fish, being one of the least studied lines of research with in ichthyoparasitology in Peru, despite being a country with a wide diversity of cartilaginous fish (Cornejo et al., 2015).
Among the 1,188 species of cartilaginous fish worldwide, holocephali or chimera fish constitute 49 species (Carrier et al., 2004; Weigmann, 2016). In the last five years, multiple aspects of parasi tology in holocephalan fishes have been studied worldwide, including studies on the structure of the parasite community as a tool to predict the population structure of its host, the discovery of new parasite species by molecular and morpho logical analysis, parasite-host phylogenetic rela tionships and the lack of geographic patterns in the distribution of their species, and their utility as bioindicators of heavy metal contamination, among others (Morris et al., 2016; Derouiche et al., 2019; Morris et al., 2019; Bray et al., 2020; Barčák et al., 2021).
The cockfish Callorhinchus calloryn chus (Linnaeus, 1758) (Chimaeriformes: Callorhinchidae) belongs to the group of carti laginous fish and is representative of the holo cephalans associated with the marine coasts. It is distributed only in the southern hemisphere, being found on the Southeast Pacific side, from northern Peru including the coast of Ecuador, to the Strait of Magellan. On the Atlantic side, it is distributed along the coast of Brazil, Uruguay, and Argentina, up to the Beagle Channel. Ca. callorynchus is a demersal marine fish, with a depth range of 10 to 116 m. It is usually found in sandy and muddy substrates; it is oviparous and according to the International Union for Conservation of Nature its conservation status is vulnerable. It is caught as bycatch and is con sumed by the local population on the marine coasts of South America (Chirichigno & Cornejo, 2001; Cousseau & Perrotta, 2000; López et al., 2000; Swing & Beárez, 2006; Bernasconi et al., 2015; Weigmann, 2016; Finucci & Cuevas, 2020; Froese & Pauly, 2021).
This species is economically important in the artisanal fishery of the town of San Andrés, Pisco, Ica, because its commercialization and hu man consumption is almost constant, despite be ing caught as bycatch. It is consumed in the form of ceviche, fish stew, and fried. Considering the commercialization of this species, there is a need to investigate the parasitology of cockfish from an ecological aspect, seeing the diversity of spe cies of parasites described in this host in studies carried out in Peru compared to other countries such as Chile and Argentina (Luque & Iannacone, 1991; Di Giácomo & Perier, 1994; Tantaleán & Huiza, 1994; Di Giácomo & Perier, 1996; Aedo et al., 2010; Luque et al., 2016; Martínez et al., 2016; Carvalho-Azevedo et al., 2021).
Therefore, the present study aimed to deter mine the characteristics of the community of par asite metazoans of the cockfish Ca. callorynchus from the artisanal fishing landing of San Andrés, Pisco, Ica, Peru, and evaluate the ecological as pects of the host-parasite and observe possible interactions among these characteristics.
MATERIALS AND METHODS
Collection and execution place. Thirty-one specimens of Ca. callorynchus were obtained from Lagunillas (Paracas Bay) and Laguna Grande (Independencia Bay) through the artis anal fishing landing of San Andrés, Province of Pisco, Department of Ica (13°44’01” S; 76°13’30” W), Peru from June 2018 to October 2019. The fish were frozen and transported to the laborato ry in Lima, Peru.
Collection of parasites. The skin, oral cavity, gills, stomach, heart, spiral valve, liver, gonads, and spleen of the collected fish were thorough ly examined. The parasitic metazoans collected were fixed in 4% formaldehyde and conserved in 70% ethanol alcohol. For identification, the para sitic metazoans were stained with Semichon ace tic carmine, dehydrated in a consecutive battery of ascending concentrations of ethanol (70%, 80%, 90% and 100%), diaphanized in eugenol and finally mounted on permanent preparations with Canada balsam (Almeida & Almeida, 2014). The copepods were preserved with alcohol-form aldehyde-acetic acid for later observation of the structures of taxonomic importance (Eiras et al., 2006). The hirudineans were fixed in 70% eth yl alcohol. Nematodes and copepods were rinsed with Amann’s lactophenol and preserved in 70% ethyl alcohol. A Euromex®-SB.1903 trinocular stereoscope was used for the observations.
Classification and determination of par asites. The identification of cestodes, mono geneans, copepods, and hirudineans parasites was based on specialized publications (Szidat, 1972; Castro & Baeza, 1984; Fernández et al., 1986; Deets, 1987; Boeger & Kritsky, 1989; Beverly-Burton et al., 1993; Oceguera-Figueroa & Pacheco-Chaves, 2012; Vaughan & Christison, 2012; Poddubnaya et al., 2015; Luque et al., 2016; Caira & Jensen, 2017; Ruiz-Escobar & Oceguera- Figueroa, 2019).
Data processing and statistical analysis. The ecological parasitological indices of preva lence (P), mean abundance (MA), and mean in tensity (MI) were estimated (Bush et al., 1997; Bautista-Hernández et al., 2015). The specific importance index (I) calculated as the impor tance of each parasitic species in the ecological assembly was used. I = Prevalence + (mean abundance [numerical or volumetric] x 100) in order to obtain an integrated infection index for both ecological descriptors (Iannacone et al., 2012). The type of strategy of each parasitic spe cies was evaluated according to the percentage of P%. Species with a prevalence higher than 45% were classified as “core” species, while those with a prevalence between 10% - 45% were de fined as “secondary” species, and species with a prevalence lower than 10% were classified as “satellite” species (Bush & Holmes, 1986).
The following indices were used: dispersion index (dI), Poulin discrepancy index (PDI) and K of the negative binomial equation with its re spective Chi square value (X2) to determine the type of distribution and degree of aggregation (Bego & Von Zuben, 2010). The Pearson correla tion coefficient (r) was used to determine the re lationship of the total body length (TBL) of the host fish with the MA and MI of each parasitic species. The Spearman correlation coefficient (rs) was applied to evaluate the association between TBL versus the prevalence of infestation, previ ously transforming the P% values to square root of arcsine. In all cases, the normality of the data was verified using the Kolmogorov-Smirnov test with the modification of Lillierfors and the ho moscedasticity of variances based on the Levene test (Zar, 2014). The Student’s t test was used to compare the MA and MI of each parasite and the sex of the host. The analysis of parasites in rela tion to TBL and host sex was performed only for species with a prevalence greater than 10% (Esch et al., 1990). 2 x 2 contingency tables were used to calculate the degree of association between the sex of the host and P% of each parasite by means of X2. Yates’ correction was used to correct for possible X2 errors with respect to the P% of parasites. For the determination of the descrip tive and inferential statistics, a p value of 0.05 was considered significant.
The following alpha dI were calculated: rich ness: species richness and Margalef, and the rich ness estimator: Chao-1; equity: Shannon-Wiener and Equitability; and dominance: Berger-Parker (Bego & Von Zuben, 2010) for the total parasit ic community component, and for males and fe males. A dendrogram with the Ward method and Euclidean index was constructed to compare the similarity between the parasites of the 31 speci mens of Ca. callorynchus studied. For the deter mination of the dI, the statistical package PAST-Palaeontological Statistics ver.4.09 was used, and for the descriptive and inferential statistics the statistical package Quantitative Parasitology 3.0 was used (Reiczigel et al., 2019).
Finally, the representative specimens of the metazoan parasite species collected were depos ited in the collection of parasitic helminths and related invertebrates belonging to the Zoological Collection of the Natural History Museum of the Universidad Nacional Federico Villarreal (UNFV) under the codes: MUFV: ZOO-HPIA: 177 - 182.
RESULTS
The Callorhinchus callorynchus population consisted of 31 specimens distributed in 13 fe males (TBL = 64.12 ± 12.85 cm) and 18 males (TBL = 55.70 ± 9.30 cm). The female population had a larger TBL than the males (t = 2.02, p <0.05). At least one parasite specimen was found in 90.32% (n = 28) of the fish. A total of 263 specimens belonging to 6 parasites species were found and identified (Table 1).
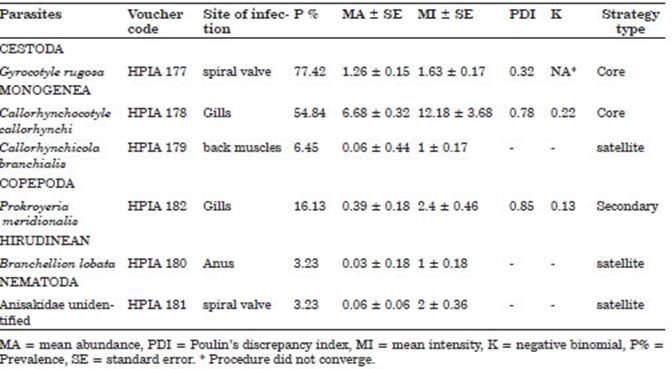
Table 1 Ecological parameters of the parasite metazoans of the cockfish Callorhinchus callorynchus from the artisanal fisheries of San Andrés, Pisco, Peru.
The three parasites with the highest preva lence of infestation and which had a P%> 10% were Gyrocotyle rugosa Diesing, 1850 (Cestoda), followed by Callorhynchocotyle callorhynchi (Manter, 1955) (Monogenea) and Prokroyeria me ridionalis (Ramírez, 1975) (Copepoda). Similarly, the parasites with the highest MA and MI of in festation were Cle. callorhynchi, followed by G. rugosa and P. meridionalis. Regarding the type of strategy, only G. rugosa and Cle. callorhynchi were considered as core species in the cockfish parasite community (Table 1). The PDI for Cle. callorhynchi and P. meridionalis showed values close to 1, and thus, the type of distribution was considered aggregate or contagious (Table 1).
The correlation coefficients of the most prev alent parasites (G. rugosa, Cle. callorhynchi, and P. meridionalis) showed no degree of association between the ecological variables of the parasites and the morphological aspects of the host (TBL and sex). There was also no significant associa tion between the sex of Ca. callorynchus and the ecological variables. The Student’s t test showed no differences between males and females of Ca. callorynchus (Table 2). Likewise, for Cle. callo rhynchi the homogeneity of variances was not fulfilled, and therefore, the Mann Whitney U test was used.

Table 2 Correlation of total body length and sex of Callorhinchus callorynchus vs. prevalence, mean abundance, and mean intensity of the most prevalent metazoan parasites.
According to the alpha dIs, higher values were observed in richness, number of individu als, and dominance (Berger-Parker) in the pop ulation of females compared to males. On the other hand, equitability and diversity (Shannon- Wiener) was biased in favor of the male popula tion of Ca. callorynchus. The sampling effort was optimal, which was corroborated with the Chao- 1 richness estimator (Table 3).
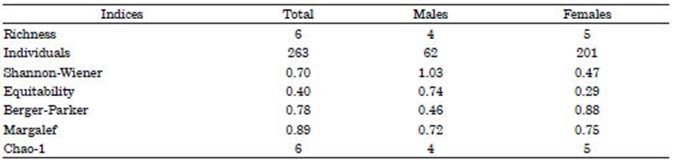
Table 3 Parasitic alpha diversity indices according to community component and sex of Callorhinchus callorynchus from artisanal fishing in San Andrés, Pisco, Ica, Peru.
A dendrogram was constructed based on Ward method and the Euclidean index to determine the similarity of the Ca. callorynchus parasites. The most similar were Anisakidae gen. et sp. in det, Callorhynchicola branchialis Brinkmann, 1952 and Branchellion lobata Moore, 1952 based on the shortest distance presented. The mono geneous Cle. callorhynchi, the most abundant of all the parasites collected, presented a great er distance in the dendrogram in relation to the rest of the parasitic metazoans, followed by the cestode G. rugosa and the copepod P. meridiona lis (Fig. 1).
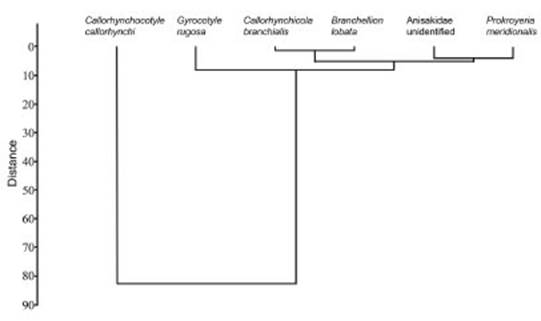
Fig. 1 Dendrogram of quantitative similarity based on Ward method and Euclidean index for the metazoan parasites of the cockfish Callorhinchus callorynchus from the artisanal fisheries of San Andrés, Pisco, Ica, Peru.
Finally, in the bibliographic search for reports of parasites in Ca. callorynchus, it was found that 13 species of metazoan parasites and three indeterminate species had been described in this host from 1927 to the present study. All reports are restricted to the southeast of the Pacific (Peru and Chile) and southwest of the Atlantic of the South American continent (Brazil, Uruguay, and Argentina). The finding of the parasites G. rugosa, B. lobata, and P. meridionalis constitutes new geographical records in Peru. Likewise, the unidentified nematode appears as a new record in the host of the cockfish (Table 4).
DISCUSSION
In the present study, the community of meta zoan parasites of Callorhinchus callorynchus was determined, thereby increasing the geo graphic distribution of some parasites and range of hosts for others. It is known that the vast ma jority of these parasites are primitive organisms in their lineages, they infest almost exclusively chimeras and have established a co-evolutionary dependence with their hosts (Caira et al., 2012).
Previous records have shown that chime ras are usually parasitized by cestodes of the Gyrocotylidae family in the spiral valve, a site that is specific for this helminth (Alves et al., 2017). The genus Gyrocotyle Diesing, 1850 is in dicated as a parasitic cestodaria restricted only to holocephalan fishes of the genus Callorhinchus (Bray et al., 2020; Barčák et al., 2021). Gyrocotyle is a group reduced to 10 valid species (Barčák et al., 2021; WoRMS, 2021). In Peru, only Gyrocotyle maximaMacDonagh, 1927 was registered par asitizing Ca. callorynchus from Lima and La Libertad (Luque et al., 2016). The present study constitutes the first record of G. rugosa from Peru. This species has been previously recorded on the marine coasts of Argentina, Chile, South Africa, and New Zealand (Reed, 2015; Barčák et al., 2021).
According to parasitological parameters, the parasite with the highest prevalence was Gyrocotyle rugosa (P% = 77.4%), making it an important and core species in the communi ty of parasites in Ca. callorynchus. A P% val ue of 71.43% has been reported for G. rugosa (Synonym of G. plana) in C. capensis from South Africa (Morris et al., 2019). In the present study, no more than one Gyrocotyle species was found in the studied host. It has been argued that most of the cockfish studied are parasitized by one or two specimens per fish and by a single (rarely two) Gyrocotyle species, which is why they are consid ered oioxenic parasites (Barčák et al., 2021).
It is suggested that Ca. callorynchus is infect ed with the G. rugosa cestode because of its ben thic diet mainly based on bivalves, brachyurans, gastropods, isopods, and anomurans (Roman et al., 2020), which are invertebrates that have pre viously been reported as intermediate hosts of several species of helminths. Gyrocotyle seems to remain within the digestive tract of its hosts for a long time and possibly throughout the life of the holocephalans, which could explain why the Gyrocotyle species were the most prevalent in south american Ca. callorynchus as well as those studied in South Africa (Halvorsen & Williams, 1968; Williams et al., 1987).
Although G. rugosa is a species with a high P%, the degree of aggregation was the lowest among the most prevalent parasites, probably due to the low number of parasitic individuals per fish. Nonetheless, the distribution of this parasite encompassed the majority of holoceph alans evaluated.
On the other hand, the monogenean Callorhynchocotyle callorhynchi presented the highest value of MA and MI in fish from Peru (MA = 6.68; MI = 12.18) which was also simi lar in C. capensis from South Africa (MA = 1.55; MI = 4.79) (Morris et al., 2019). This is due to the greater number of specimens per host, which varied from 1 to 61 individuals of Cle. callorhyn chi for each cockfish. It is known that the MI index is higher when a population of parasites is high in a small group of hosts, which is why it is argued that the species Cle. callorhynchi is characterized by presenting this high value in the host populations.
Many studies on cockfish carried out in Peru have registered the monogeneans Callorhynchocotyle marplatensis Suriano & Incorvaia, 1982 (Luque & Iannacone, 1991; Tantaleán & Huiza, 1994), Cla. branchialis (Martínez et al., 2016), and Cle. callorhynchi (Carvalho-Azevedo et al., 2021) in the gills of cockfish, all from the town of Chorrillos, Lima. In this study, Cle. callorhynchi and Cla. branchi alis were registered for the second time in the same host but were registered for the first time in the department of Ica. In the mentioned sur veys, both species of monogenean were evaluat ed qualitatively and descriptively, and therefore, this is the first determination of the quantitative nature of this population of parasites in Peru.
The third species considered important in this community of parasites was the population of Prokroyeria meridionalis, which had a secondary category due to the prevalence presented (P% = 16.13%). This host-parasite association has been previously described in Argentina and Chile and now for the first time in Peru (Ramírez, 1975; Castro & Baeza, 1984; Fernández et al., 1986; Deets, 1987; Castro-Romero et al., 2016). The MI of infestation of this species was the second highest (MI = 2.4 ± 0.46) recorded in this study, similar to that observed in G. rugosa. Likewise, it was the species with the highest degree of ag gregation (DOA = 0.85).
Branchellion Savigny, 1822 is a genus that includes blood-sucking leeches of strictly marine hosts, with a preference for batoid fish to which they adhere to the surface and / or body orifices (Rohde, 2005; Caira et al., 2012). In the Eastern Pacific, the species B. lobata and B. callorhynchusSzidat, 1972, both in Chile, are qualitatively re ported (Szidat, 1972; Ringuelet, 1985; Fernández et al., 1986). In the present study, only one indi vidual of B. lobata was found in the anal orifice of a cockfish, making it difficult to determine the behavior of this species in Ca. callorynchus.
In our study, no correlation was observed between the TBL of the fish and the P%, MA, and MI of the parasites Gyrocotyle rugosa, Cle. callorhynchi, and Prokroyeria meridionalis. In contrast, Morris et al. (2019) found a correlation between the abundance of Cle. callorhynchi and the TBL and weight of C. capensis. Additionally, these same authors found a significant positive relationship between the weight of C. capensis and the abundance of G. rugosa. The findings of Morris et al. (2019) suggest that smaller fish con tain a lower abundance of parasites compared to larger fish. This idea is reinforced by Poulin (2011) who indicated that the largest hosts can provide a greater supply of nutrients to the par asites and, consequently, they would be the most susceptible to a greater parasite diversity and load. In our case, the lack of association between the parameters evaluated indicates that other factors may influence the population dynamics of the parasites, such as host infection at an early age. Halvorsen & Williams (1968) speculated that Gyrocotyle infections begin when young hosts be gin to feed, which was also observed in this study as fish with a shorter total length (33.1 cm) pre sented a moderately elevated parasitic infection indicating that the infection begins in the early stages of life.
According to the alpha dIs, the Berger Parker dominance index showed low values of parasites found in male fish and high values in females (0.46 and 0.88, respectively) and consequently, a greater diversity in the population of males. Iannacone et al. (2012) argue that the selection of parasites by either sex of the host fish could be attributed to differences in the ecological relationships (habitat, behavior, and feeding) of males and females (Minaya et al., 2020c). Regarding the Chao-1 richness estimator, the number of parasitic species present in the sam pled Ca. callorhynchus, in both males and fe males and the total population (males + females) showed a richness similar to that estimated by this index. Therefore, the sampling effort was optimal for the 31 specimens collected.
Addressing the historical aspects in the parasitological studies in Ca. callorynchus, Fernández et al. (1986) found the largest num ber of species in a single population of cockfish from central-southern Chile to date, describing nine species of parasites (Table 4). Of these, six corresponded to phylogenetic type parasites con ditioned by historical and zoogeographic factors: Cla. branchialis, Cle. callorhynchi, Multicalyx elegans (Olsson, 1869), G. rugosa, G. maxima, and P. meridionalis. The other three species corresponded to ecologically-acquired para sites: Branchellion callorhynchus, Caligus teres Wilson, 1905 and Ceratothoa sp.
CONCLUSION
The three parasites with the highest prev alence of infestation were Gyrocotyle rugosa (Cestoda), followed by Callorhynchocotyle callo rhynchi (Monogenea) and Prokroyeria meridionalis (Copepoda). Similarly, the parasites with the highest MA and MI of infestation were Cle. callorhynchi, followed by G. rugosa and P. me ridionalis. Regarding the type of strategy, only G. rugosa and Cle. callorhynchi were considered as core species in the cockfish parasite commu nity. The sampling effort was optimal, which was corroborated with the Chao-1 richness es timator. This work provides the first extensive quantitative analysis of the parasitic community in the cockfish Ca. callorynchus in Peru, as well as three new geographic records for G. rugosa, P. meridionalis, and B. lobata in Peru and a new record for a host of a nematode infesting Ca. cal lorynchus.












 uBio
uBio 


