Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
On-line version ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.45 no.2 Mendoza Dec. 2013
ARTÍCULOS ORIGINALES
Minimum water input event for seedling emergence of three native perennial grasses of the Central Monte desert (Argentina) influenced by the effect of shade and the season of the year
Pulso mínimo de agua para la emergencia de plántulas de tres especies de gramíneas perennes nativas del Monte Central (Argentina), influenciado por el efecto de la sombra y la estación del año
Silvina A. Greco 1, Carmen E. Sartor 1, Pablo E. Villagra 1, 2
1 Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Alte. Brown 500. Chacras de Coria. Mendoza. Argentina. M5528AAHB. sgreco@fca.uncu.edu.ar
2 Dpto. de Dendrocronología e Historia Ambiental. Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales (IANIGLA), CCT Mendoza - CONICET. C. C. 330. (5500). Mendoza, Argentina.
Originales: Recepción: 15/06/2012 - Aceptación: 15/06/2013
Abstract
In deserts, seedling emergence occurs only after precipitation threshold has been exceeded, however, the presence of trees modifies microenvironmental conditions that might affect the effectiveness of a water pulse. In the Monte desert, Prosopis flexuosa trees generate different micro-environmental conditions that might influence grass seedlings establishment. The objective of this work was: a) to know the effective minimum water input event that triggers the emergence of native perennial grass seedlings; b) to relate this fact with the effect of the shade of P. flexuosa canopy and the seasonal temperatures. Three important forage species of the Monte were studied: Pappophorum caespitosum and Trichloris crinita, with C4, and Jarava ichu, with C3 metabolism. Each season, seeds of these species were sown in pots placed at two light conditions: shade (similar to P. flexuosa cover) and open area, and with seven irrigation treatments (0, 10, 20, 30, 40, 2*10 and 3*10 mm). J. ichu did not emerge in any of the treatments. Significant seedling emergence was registered for P. caespitosum and T. crinita in shade conditions with 40 mm irrigation treatment in summer. Since 40 mm precipitation events are infrequent in the Monte, seedling emergence for these species would be restricted to exceptional rainy years. The facilitating effect of P. flexuosa shade would be important during the hot season.
Keywords: Monte Desert; Perennial grasses; Water requirement; Facilitation; Seedling emergence; Shade; Season
Resumen
En los desiertos, la emergencia de las plántulas se produce cuando un evento de precipitación excede un valor umbral. La presencia de árboles modifica las condiciones micro ambientales y en consecuencia, la efectividad de los eventos de lluvia. En el Monte, las condiciones generadas por Prosopis flexuosa (algarrobo) afectarían el establecimiento de las gramíneas. El objetivo del trabajo fue: a) conocer el evento mínimo de agua necesario para desencadenar la emergencia de gramíneas perennes; b) relacionarlo con la influencia de la sombra del algarrobo y las temperaturas estacionales. Se trabajó con tres especies nativas de gramíneas forrajeras perennes: Pappophorum caespitosum, Trichloris crinita, (C4), y Jarava ichu (C3). En cada estación del año, semillas de las tres especies fueron sembradas en macetas bajo dos condiciones lumínicas: sombra (similar a la sombra de P. flexuosa) y área abierta y con siete niveles de riego (0, 10, 20, 30, 40, 2*10 y 3*10 mm). Jarava ichu no emergió en ningún tratamiento. En el verano se obtuvo emergencia significativa de T. crinita y P. caespitosum solo en los tratamientos de 40 mm y a la sombra. Debido a que en el Monte los eventos de precipitación de 40 mm son infrecuentes, la emergencia de estas especies ocurriría en los años excepcionalmente lluviosos. El efecto facilitador de la sombra del algarrobo sería de importancia en la estación cálida.
Palabras claves: Desierto del Monte; Gramíneas perennes; Requerimiento hídrico; Facilitación; Emergencia de plántula; Sombra; Epoca del año
Introduction
In deserts, rainfall events are highly variable and largely unpredictable (20). The magnitude of a rainfall event (or pulse) and the length of time that water remains on soil trigger different biological responses (27). Usually, this relationship has a minimum size of the event or "threshold" under which a specific biological process does not occur; however, soil type, climate and vegetation characteristics modify the pulse effectiveness (27).
Germination and establishment in deserts occur only after the precipitation threshold has been exceeded (4, 21), when temperatures are appropriate for these processes (12). This threshold has a specific nature (17) and is lower for winter germinating species than for summer species (14). These differences may be related to the way that temperature influences soil evaporation rate. At micro-environmental scale, trees and shrubs cover also reduces the soil evaporation rate (2, 6) increasing the effectiveness of a water pulse to trigger germination (1).
The Central district of the Monte Desert is characterized by an arid environment with mean annual rainfall between 120 and 350 mm (24). More than 75% of annual rainfall occurs from October to March (spring / summer months) (23). During the rainy season, rainfall events are unpredictable and most of them less than 10 mm. For example in the Biosphere Reserve of Ñacuñán (34°03'5 S, 67°55' W) out of 1011 rain events recorded during 15 years, 82% were lower than 10 mm, 12% from 10 to 24,9 mm, and only 6% higher than 25 mm (figure 1, page 199).
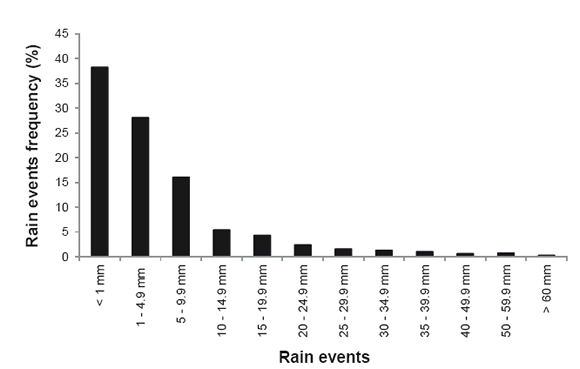
Figure 1. Rain-event frequency of different precipitation pulses in Ñacuñán Reserve from 1990 to 1998, and from 2000 to 2007. Each event represents the cumulative rainfall in a day.
Figura 1. Frecuencia de los eventos de precipitación de distinta intensidad registrados en la Reserva de Biosfera de Ñacuñán desde 1990 hasta 1998 y desde el 2000 hasta el 2007. Cada evento representa la precipitación acumulada en un día.
In this desert, perennial grasses species are the main component of the herbaceous layer. Most of these grasses are C4 metabolism species and Jarava ichu is the only one with C3 metabolism (29). Perennial grasses emerge throughout the rainy season but emergence is very low (less than 1% of soil seed bank) and highly influenced by the amount of precipitation during the season (18). This pattern suggests that the emergence of these species is triggered after the adequate rainfall threshold for the subsequent seedlings survival is reached (5).
Besides, the presence of Prosopis flexuosa tree causes a difference in water, thermal and edaphic conditions and in some ecosystems processes regarding to open areas and therefore, it determines a different spatial distribution of grasses (9, 19, 26). This distribution varies depending on the location of the Central Monte desert.
In the Telteca Reserve (32°19' S, 67°54' W) with an average annual rainfall of 150 mm and average annual temperature of 18.2°C (10), the forage grasses Trichloriscrinita and Pappophorum caespitosum are common under the tree canopy (8) whereas in Ñacuñán Biosphere Reserve, with an average annual rainfall of 335 mm and average annual temperature of 15.6°C (11), they are usually found in open areas (25, 26). Jarava ichu is found only in Ñacuñán and always under the Prosopisflexuosa canopy (26).
The aim of this work was to determine under semi-controlled conditions: a) the minimum water input event for the seedling emergence of three important native forage grasses species of the Monte: Pappophorum caespitosum, Trichloris crinita and Jaravaichu in different seasons over the year; b) the influence of a shade similar to P. flexuosa canopy on the effectiveness of a water input event to trigger grass seedlings emergence.
Materials and methods
Species
The experiment was conducted with three native perennial forage grass species of the Central Monte, Pappophorum caespitosum R. E. Fr. and Trichloris crinita (Lag) Parodi (29), with spring-summer cycle and C4 metabolism; and Jarava ichu Ruiz and Pav with winter-spring cycle and C3 metabolism. Seeds were gathered in Ñacuñán Biosphere Reserve between April and June of years 2005 and 2006.
Experimental design
The trial was carried out at the experimental site located in the Facultad Ciencias Agrarias, Universidad Nacional de Cuyo (Chacras de Coria, Mendoza, Argentina, 32°59' S and 68°52' W).
In order to protect the trial against rain and hail, an iron structure of 8 m long and 6 m wide with a roof made by 200 micron-Cristal polyethylene and white hail protection net were used. This structure was divided into two different light conditions: the open area that was protected only with the polyethylene and the hail net; and the shade treatment that was also covered with a black shade net with 65% reduction of radiation intensity. Radiation intensity measured under shade treatment at noon was 400 μ Einstein m-² s-¹ during summer, which matched with the radiation values registered by Rossi (25) under Prosopis flexuosa canopy in Ñacuñán.
A full randomized design was applied within each light condition plot, with five replicates of each irrigation treatment and each of the three grass species (7 irrigation treatments x 3 grass species x 5 replicates). The experiment was repeated four times during a year: December 2005 (summer), April (autumn), August (winter) and November (spring), 2006.
Seven irrigation treatments of 0, 5, 10, 15, 20, 30 and 40 mm of water were applied in December. The amount of water applied for each treatment was calculated according to the area of the pot. Water was applied as rainfall. In December trial, seedling emergence was recorded only in 40 mm irrigation treatment, so 5 and 15 mm treatments were replaced in the rest of the trials by two other treatments: two days in a row with 10 mm irrigation (2*10 mm), and three days in a row with 10 mm irrigation (3*10 mm) in order to simulate two and three days of precipitation with little amount of rainfall every day. Therefore, in April, August and November trials, the irrigation treatments were: 0, 10, 20, 30, 40, 2*10 and 3*10 mm.
Thirty-five seeds of each species were sown into 200 micron-black polyethylene pots with 10 cm diameter and 40 cm depth. These pots were filled with sterilized sandy soil which contained 0,48% organic matter and 301 mg.kg-1 total nitrogen, similar to the open areas found in the Monte (26). Sowing depth was 0.5 cm, as determined by Tironi et al. (28) for these species.
Seedling emergence was recorded daily and new seedlings were marked. Each trial lasted 15 days. The emergence percentage was calculated as the total number of seedlings emerged divided by the number of 35 seeds and multiplied by 100.
In order to monitor water evaporation from the soil, we measured soil water content in four pots of each water treatment for both light conditions. Each pot was weighed using a digital scale (Kretz single) before irrigation, immediately after irrigation and the following five days.
Air temperature for both light conditions and the temperature outside the structure were measured at midday every two days using a LCD Digital "Prima Long" thermometer with stainless steel sensor in December trial. Air temperature was measured with HOBO Onset sensors which were placed on the surface of one pot placed in the center of each light treatment in April, August and November trials.
Radiation intensity was measured with a Licor radiometer (LI-185 A Lincoln, Nebraska, USA) in both light conditions and outside the structure.
At the beginning of every trial a seed germination test for each species was performed by placing 25 seeds in Petri dishes, which were prepared with a layer of cotton and filter paper moistened with distilled water. Petri dishes were placed in darkness at 30°C temperature. Four replicates of each species were used and germination was recorded during 10 days.
Statistical analysis
Differences in seedling emergence among irrigation treatments for each light condition in every season were tested by Kruskal-Wallis test for each species. The soil water content among irrigation treatments for every sampling date were compared by ANOVA analysis and then by Tukey test (30).
Results
Seeds of the three species showed high germination percentages in laboratory tests that ranged from 76 to 81% for Pappophorum caespitosum, 73 to 94% for Trichloris crinita and 82 to 95% for Jarava ichu.
December 2005
P. caespitosum and T. crinita emerged only in 40 mm irrigation treatment (figure 2, page 202) in shade conditions. Emergence of P. caespitosum was 30 ± 10% and emergence of T. crinita was in 19.6 ± 9.4%. T. crinita emerged only in 40 mm irrigation treatment 3.6 ± 3.6% and P. caespitosum did not emerge in any water treatment in open area. J. ichu did emerge neither in light conditions nor in irrigation treatments.
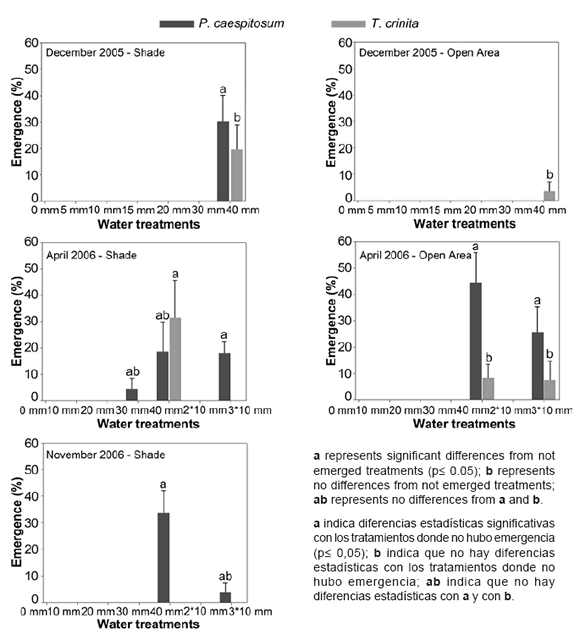
Figure 2. Seedling emergence percentage (+ Standard Error) of Pappophorum caespitosum (black bars) and Trichloris crinita (grey bars) under shade (left) and open areas treatments (right) in December, April and November trials. No emergence was registered in August in any treatment and in November in the open area treatment. Statistical analysis compared among water treatments for each light condition for P. caespitosum and T. crinita separately.
Figura 2. Porcentaje de plántulas (+ Error Estándar) de Pappophorum caespitosum (barras negras) y Trichloris crinita (barras grises) emergidas bajo condiciones de sombra (izquierda) y área abierta (derecha) en los ensayos de diciembre, abril y noviembre. No se observó emergencia de plántulas en agosto en ninguno de los tratamientos y en noviembre en el tratamiento de área abierta. En el análisis estadístico se compararon los tratamientos de riego entre sí para cada tratamiento de luz por separado y para cada una de las especies P. caespitosum y T. crinita separadamente.
Soil water content in 40 mm treatment was higher than in the rest of water treatments until the 4th day after irrigation (p<0.05) (figure 3, page 203). It is important to notice that in 5 and 10 mm treatments water content was zero on the 2nd day after irrigation. Air temperatures between 12:00 pm and 1:00 pm ranged between 28°C and 35°C in shade treatment, and between 35°C and 45°C at the same time in the open area treatment (figure 4, page 204).
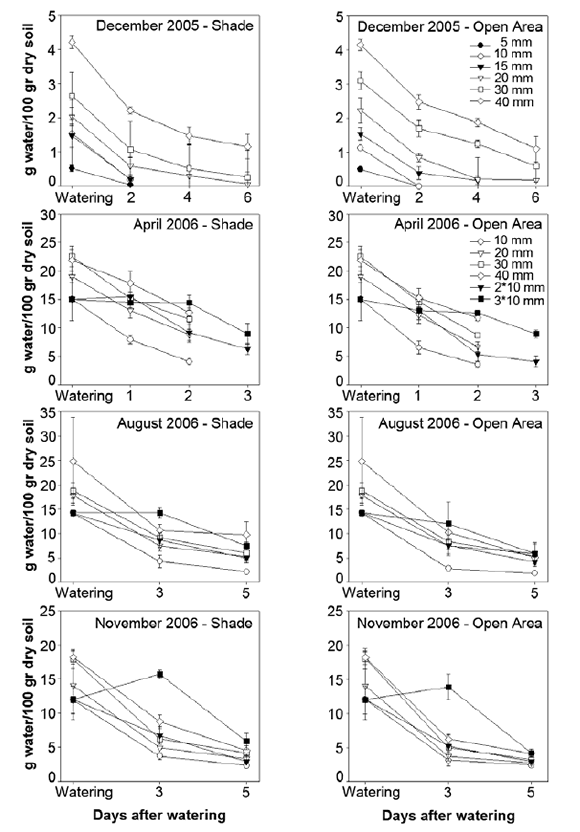
Figure 3. Soil water content (g water/100 g dry soil) (+ Standard Error) of different water treatments shown in open areas (right) and in shade (left) in December 2005, April, August and November 2006. References of water treatments are equal for both light conditions.
Figura 3. Contenido de agua del suelo (g de agua/100 g de suelo seco) de los distintos tratamientos de riego en las áreas abiertas (derecha) y en la sombra (izquierda) en diciembre del 2005, abril, agosto y noviembre del 2006. Las referencias de los tratamientos de riego son iguales para ambos tratamientos de luz.
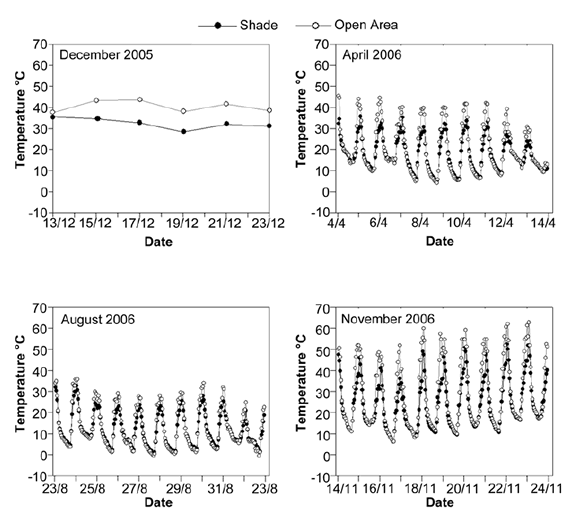
Figure 4. Midday temperatures in December and daily temperatures in April, August and November 2006 in open area (light lines) and shade (dark lines).
Figura 4. Temperaturas al medio día del ensayo de diciembre del 2005 y temperaturas diarias de los ensayos de abril, agosto y noviembre del 2006 en las áreas abiertas (líneas claras) y en la sombra (líneas oscuras).
April 2006
In shade conditions the emergence of P. caespitosum recorded in 40 mm treatment was19 ± 11%, in 3*10 mm treatment was18 ± 5 % and in 30 mm treatment was 4 ± 4% (figure 2, page 202), but only 3*10 mm treatment was significantly different from those treatments with no emergence. T. crinita emerged only in 40 mm water treatment and the percentage was 31 ± 14%.
In the open area, P. caespitosum significantly emerged in 40 mm and 3*10 mm water treatments, reaching higher values than in shade treatments (44 ± 11% and 26 ± 10% respectively). T. crinita emergence was 8 ± 5% in 40 mm treatment and 7 ± 7% in 3*10 mm treatment, but did not differ significantly from those treatments with no emergence. J. ichu did not emerge in any treatment.
On the first day after irrigation, soil water content for 40 mm treatment was the highest, even higher than for 3*10 mm. However, on the 2nd day after irrigation 40 mm and 3*10 mm treatments showed the same water content (p<0.05) (figure 3, page 203).
This pattern is observed in shade and open area treatments. In this month, the daily maximum air temperatures of open area treatment were about 8 to 10°C higher than in shade treatment. For example, temperature ranged between 28°C and 32°C in shade treatment and between 30°C and 40°C in open area treatment between 12:00 pm and 1:00 pm (figure 4, page 204).
August 2006
No emergence was recorded for any of the species in this experiment. Soil water content was higher than that measured in December, April and November trials (figure 3, page 203). Minimum temperatures were lower than 10°C for both light treatments during the whole experiment period, and there were several days with minimum temperature below zero. Maximum temperatures were higher than 30°C only four days during this trial (figure 4, page 204).
November 2006
Only P. caespitosum emerged in the 40 mm and 3*10 mm water shade treatments, but its emergence was only significant in 40 mm irrigation treatment. None of the three species emerged in open area (figure 2, page 202).
In both light conditions, soil water content on the 2nd day after irrigation was higher for 3*10 mm treatment when compared to the other irrigation treatments(p<0.05) (figure 3, page 203). However, on the 5th day all irrigation treatments showed the same soil water content. In this trial the maximum air temperatures were higher than in the others, for example, daily maximum air temperatures were about 55 - 60°C in open area treatment and about 40 - 50°C in shade treatment (figure 4, page 204). Besides, minimum temperature dropped to 5°C on the 3rd day.
Discussion
According to the results of these experiments the minimum water input event necessary for the emergence of the perennial native C4 grasses Pappophorum caespitosum and Trichloris crinita seedlings would be 40 mm of water in a sandy soil in the hot season. However, rainfall events of this magnitude are very infrequent in Central Monte desert (figure 1, page 199). For instance, for the last 15 years, out of 1011 rain events registered in Ñacuñán, only 17 events were greater than 40 mm, and only 3 events were over 60 mm (figure 4, page 204).
These results are in accordance with previous studies suggesting that seedling emergence of native perennial grasses of the Monte depends on exceptionally rainy years (18). Studies in other arid areas around the world arrived to similar conclusions (3, 12, 15). In Nuevo Mexico desert, where precipitations are mainly in summer, germination records show that the effective precipitation is, at least, 20 mm of water (16). It has been suggested that these high amounts of water necessary to trigger emergence of desert plants depend on the amount of water needed not only to trigger germination, but also to ensure survival of seedlings, at least in short term (5).
Perkins & Owens (22) observed that the establishment of Setaria and Aristida grasses is related by abnormal periods of heavy precipitations. One demographic consequence of rare events of effective precipitations is the fact that perennial populations of the desert species could be made up of cohorts established in successful years, with voids between them (17). This specific aspect has not been studied yet for perennial grasses populations of the Monte.
It must be noticed that in summer, emergence would be limited not only by low soil water content but also by direct effect of temperature. In open areas, soil temperatures are usually above 35°C and can reach 60°C at noon (25), while it has been observed that the germination of P. caespitosum and T. crinita diminishes significantly at 40°C and does not occur at 50°C.
The optimum germination temperature for both species ranges between 25°C and 35°C (13). Therefore, micro-habitats such as those generated by trees or shrubs which enrich soil water availability and reduce soil temperature would take special significance on Trichloris crinita and Pappophorum caespitosum emergence.
In contrast, in early autumn air temperatures are lower, which implies a decrease in water evaporation, so, small and daily water events could be as effective as a big one (there was a similar emergence on 3*10 mm and 40 mm treatments in April).
However, soil temperatures in open areas are more suitable for emergence than under tree canopy. Therefore, these autumn conditions increase the chance of emergence in the open areas.
Although soil water content in the winter trial (August) was higher regarding the other trials, and temperatures ranged 25°C to 30°C during daylight, emergence was recorded neither for Pappophorum caespitosum nor for Trichloris crinita, which might be owed to the fact that minimum temperature ranged between 5°C and 0°C. Germination in these species did not occur under laboratory conditions with equal or lower 5°C temperatures (13).
In the same way, during November trial, Trichloris crinita germination also might have been affected by temperatures close to 5°C which occurred on the 3rd day. Pappophorum caespitosum germination, instead, was not affected. This, probably, was due to the fact that Pappophorum caespitosum has higher germination capacity than Trichloris crinita (13), which could have allowed P. caespitosum seeds to germinate before the 3rd day.
Differences observed in Pappophorum caespitosum and Trichloris crinita emergence under shade and open area conditions are consistent with the idea that micro-climatic modifications found in different patches generated by the shade of the Prosopis flexuosa canopy can be a determining factor in the emergence and the consequent spatial distribution of species of these native grasses.
The facilitating effect of P. flexuosa shade would be significant in those months with higher temperatures and in drier places. This agree with the different spatial distribution of these species in areas with different precipitation in the Central Monte; in fact, both species prefer to grow under canopy microhabitats in drier areas (Telteca) while they prefer to grow in open areas in more humid ones (Ñacuñán) (8, 24).
Jarava ichu, the only C3 grass of the area, did not emerge in any of the field trials regardless the time of the year, although, a high percentage of germination of the seeds used in the laboratory trials was recorded. This fact indicates that this species seedling emergence needs more than 40 mm of water under field conditions. This is in accordance with C3 species characteristics, which need more humid conditions than C4 species (7).
The infrequent nature of effective water input in the Monte desert suggests a high importance of microhabitats for the establishment of perennial grasses. Particularly, in the case of J. ichu, microhabitats that accumulate water would be necessary for its emergence.
Therefore, micro topographic analysis will be necessary to understand the site conditions that favor the establishment of this species.
Conclusions
Perennial grasses of the Central Monte need very infrequent water input events to trigger seedling emergence. In spring and in summer shade conditions are more suitable for seedling emergence in drier areas, whereas, in autumn and more humid places, the open areas are more appropriate. This emphasizes the high importance of microhabitats for the establishment of the studied species.
1. Aguiar, M. R.; Sala, O. E. 1999. Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends in Ecology and Evolution 14: 273-277. [ Links ]
2. Armas, C.; Pugnaire, F. I. 2005. Plant interactions govern population dynamics in a semi-arid plant community. Journal of Ecology 93: 978-989. [ Links ]
3. Baskin, C. C.; Baskin, J. M. 2001. Seeds. Ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego. 668 p.
4. Beatley, J. 1974. Phenological events and their environmental triggers in Mojave desert ecosystems. Ecology 55: 856-863. [ Links ]
5. Bewley, D. J.; Black, M. 1994. Seeds. Physiology of development and germination. Plenum Press. New York and London. 445 p.
6. Brooker, R. B.; Maestre, F. T.; Callaway, R. M.; Lortie, C. L.; Cavieres, L. A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J. M. J.; Anthelme, F.; Armas, C.; Coll, L.; Crocket, E.; Delzon, S.; Forey, E.; Kikvidze, Z.; Olofsson, J.; Pugnaire, F.; Quiroz, C. L.; Saccone, P.; Schiffers, K.; Seifan, M.; Touzard, B.; Michard, R. 2008. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology 96(1): 18-34.
7. Cavagnaro, J. B. 1988. Distribution of C3 and C4 grasses at different altitudes in a temperate arid region of Argentina. Oecologia 76: 273- 277. [ Links ]
8. Cesca, E. 2003. Efecto de Prosopis flexuosa sobre las especies palatables y su importancia para el manejo pastoril de los algarrobales del noroeste de Mendoza. Tesis de Grado. Universidad de Congreso. p. 61.
9. Cesca, E. M.; Villagra, P. E.; Passera, C.; Alvarez, J. A. 2012. Effect of Prosopis flexuosa on understory species and its importance to the pastoral management of woodlands in the Central Monte Desert. Rev. FCA UNCUYO. 44(2): 207-219. [ Links ]
10. Estrella, H. A.; Heras, V. A.; Guzzetta, V. A. 1980. Registro de elementos climáticos en áreas críticas de la Provincia de Mendoza. Cuaderno Técnico IADIZA 2: 49-72.
11. Estrella, H.; Boshoven, J.; Tognelli, M. 2001. Características del clima regional y de la Reserva de Ñacuñán. En: El desierto del Monte: La Reserva de Biosfera de Ñacuñán, S. C. y. S. Roig- Juñent, ed. IADIZA-CONICET, Mendoza. p. 25-34.
12. Freas, E. K.; Kemp, P. R. 1983. Some relationships between environmental reliability and seed dormancy in desert annual plants. Journal of Ecology 71: 211-217. [ Links ]
13. Greco, S. A.; Cavagnaro, J. B.; Marone, L. 2003. Efecto de la temperatura en cuatro gramíneas forrajeras del Monte. Boletín de la Sociedad Argentina de Botánica 38: 182.
14. Grime, J. P. 1981. The role of seed dormancy in vegetation dynamics. Annals of Applied Biology 98: 555-558.
15. Gutterman, Y. 1990. Do germination mechanisms differ in plants originating in desert receiving winter or summer rain? Israel Journal of Botany 39: 355-372.
16. Herbel, C. H.; Gibbens, R. G. 1989. Matric potential of clay loam soils in arid rangeland in southern New Mexico. Journal of Range Management 42: 386-392. [ Links ]
17. Kigel, J. 1995. Seed germination in arid and semiarid regions. In: Seed Development and Germination, K. Galili, ed. Dekker Inc. New York-Hong Kong. p. 645-699.
18. Marone, L.; Horno, M. E.; González del Solar, R. 2000. Post dispersal fate of seeds in the Monte desert of Argentina: patterns of germination in successive wet and dry years. Journal of Ecology 88: 940-949.
19. Miner, A.; Villagra, P.; Alvarez, J.; Aranibar, J. 2010. Dinámica temporal de la masa de broza en distintos microhábitats del desierto del Monte Central (Mendoza, Argentina). Rev. FCA UNCUYO. 42(2): 55-69.
20. Noy-Mer, I. 1973. Desert ecosystems: environment and producers. Annals Review of Ecology and Systematics 4: 25-52. [ Links ]
21. Noy-Mer, I. 1974. Desert ecosystems: higher trophic levels. Annual Review of Ecology and Systematics 5: 195-214.
22. Perkins, S.; Owens, M. K. 2003. Growth and biomass allocation of shrub and grass seedlings in response to predicted changes in precipitation seasonality. Plant Ecology 168: 107-120.
23. Pol, R. G.; Pirk, G. I.; Marone, L. 2010. Grass seed production in the central Monte desert during successive wet and dry years. Plant Ecology 208: 65-75. [ Links ]
24. Roig, F.; Roig-Juñent, S.; Corbalán, V. 2009. Biogeography of the Monte desert. Journal of Arid Environments 73: 164-172.
25. Rossi, B. E. 2004. Flora y vegetación de la Reserva de Biosfera de Ñacuñán después de 25 años de clausura. Heterogeneidad espacial a distintas escalas. Tesis Doctoral Universidad Nacional de Cuyo, Mendoza. 151 p.
26. Rossi, B. E.; Villagra, P. E. 2003. Effects of Prosopis flexuosa on soil properties and the spatial pattern of under storey species in arid Argentina. Journal of Vegetation Science 14: 543-550.
27. Schwinning, S.; Sala, O. E. 2004. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141: 211-220. [ Links ]
28. Tironi, C.; Sartor, C. E.; González del Solar, R. 2003. Germinación y emergencia de dos gramíneas perennes del Monte central en respuesta al enterramiento. En: XXIX Jornadas Argentinas de Botánica y XV Reunión Anual de la Sociedad Botánica de Chile, San Luis. Vol. 38. p. 199.
29. Villagra, P. E.; Giordano, C. V.; Alvarez, J. A.; Cavagnaro, J. B.; Guevara, A.; Sartor, C. E.; Passera, C. B.; Greco, S. 2011. Ser planta en el desierto: estrategias de uso de agua y resistencia al estrés hídrico en el Monte Central de Argentina. Ecología Austral 21: 29-42. [ Links ]
30. Zar, J. H. 1999. Biostatistical analysis. Prentice Hall, Upper Saddle River, New Jersey. 663 p.
Acknowledgments
To G. Greco and A. Caretta for English translation.
M. S. Nievas, M. Sartor and M. A. Talaguirre for technical assistance.
This work was supported by Secretaría de Ciencia, Técnica y Posgrado de la Universidad Nacional de Cuyo (Argentina).














