Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
versión On-line ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.46 no.2 Mendoza dic. 2014
ARTICULO ORIGINAL
Quantitative micrograph, HPLC and FTIR profiles of Melissa officinalis and Nepeta cataria (Lamiaceae) from Argentina
Micrografía cuantitativa y perfiles de HPLC y FTIR de Melissa officinalis y Nepeta cataria (Lamiaceae) de Argentina
Marta E. Petenatti 1, 2, María A. Gette 2, Gerardo E. Camí 4, Mariana C. Popovich 2, Eduardo J. Marchevsky 5, Luis A. Del Vitto 1, 2, Elisa M. Petenatti 1, 3
1 Herbario de la Universidad Nacional de San Luis/Proy. 22/Q-016 SPU-ME. Ejército de los Andes 950, D5700HHW, San Luis, Argentina. mepetena@unsl.edu.ar
2 Cátedra de Farmacobotánica, Facultad de Química, Bioquímica y Farmacia.
3 Cátedra de Farmacognosia, Facultad de Química, Bioquímica y Farmacia.
4 Laboratorio de Química Inorgánica, Facultad de Química, Bioquímica y Farmacia.
5 Instituto de Química de San Luis/CONICET.
Originales: Recepción: 10/12/2013- Aceptación: 10/06/2014
ABSTRACT
Melissa officinalis L., so called "Melissa" or "Toronjil", is a perennial aromatic herb, whose leaves are used in traditional medicine as a carminative, digestive and sedative, both in simple as in mixtures. Meanwhile, Nepeta cataria L., commonly called "Cat mint" or "Toronjil", with some similar properties, often replacing M. officinalis in the market, although their chemical composition is not completely matched, and contains an iridoid potentially toxic (nepetalactone). It is therefore necessary to establish diacritic parameters to differentiate these species, both at crude drug level, mixtures and extracts. Samples from various sources in Argentina were studied and documental specimens are preserved in the Herbarium UNSL. Anatomical sections were analyzed, and quantitative micrographic parameters were obtained, together with HPLC and FTIR spectra from methanolic and aqueous lyophilized extracts. Significant differences were detected in the prevailing smell foliage, morphology of leaves and inflorescences, trichomata type, palisade ratio, veinlet termination number, rosmarinic acid concentration (with distinctive HPLC profiles), and the CO/CH relationships obtained by FTIR from the extracts, that together allow adequate differentiation of both drugs, even when they were ground or powdered.
Keywords: Melissa officinalis; Melissae folium; Nepeta cataria; HPLC; FTIR; Quantitative micrographic parameters
RESUMEN
Melissa officinalis L., llamada vulgarmente "melisa" o "toronjil", es una hierba perenne, aromática, cuyas hojas son empleadas en medicina popular como carminativo, digestivo y sedante, tanto en droga simple como en asociación. Por su parte Nepeta cataria L., llamada vulgarmente "menta de los gatos" y también "toronjil", con algunas propiedades similares, sustituye con frecuencia a M. officinalis en el mercado, aunque su composición química no es del todo coincidente y contiene un iridoide potencialmente tóxico (nepetalactona). Por ello es necesario establecer parámetros diacríticos para diferenciar ambas especies, a nivel de droga cruda, mezclas y extractos. Fueron estudiados especímenes de diversas procedencias en Argentina, y los ejemplares documentales son conservados en el Herbario UNSL. Se analizaron cortes anatómicos, se registraron los parámetros micrográficos cuantitativos y se obtuvieron espectros de HPLC y FTIR a partir de extractos metanólicos y acuosos liofilizados. Fueron detectadas diferencias significativas entre ambas especies en cuanto al aroma prevaleciente del follaje, la exomorfología de hojas e inflorescencias, los tipos tricomáticos, la relación de empalizada, el número de terminales de nerviación, la concentración de ácido rosmarínico (con perfiles HPLC distintivos), y las relaciones CO/CH obtenidas por FTIR a partir de los extractos, caracteres que en conjunto permiten una diferenciación adecuada de ambas drogas, aún cuando se presenten molidas o reducidas a polvo.
Palabras clave: Melissa officinalis; Melissae folium; Nepeta cataria; HPLC; FTIR; Parámetros micrográficos cuantitativos
INTRODUCTION
The establishment of quality standards for medicinal drugs of plant origin has been emphasized by World Health Organization and other authorities (4, 33). Despite their "natural" origin, some plants are not entirely safe showing both adverse effects and interactions with other herbs, supplements, medicines, foods and even laboratory diagnostic tests (10, 13, 35). The quality, safety and efficacy have to be determined carefully by chemical complexity and intrinsic biological variation of these drugs (32, 36) in particular with reference to numerous plant species used in the treatment of central nervous system disorders (6).
Melissa officinalis L. has been incorporated into many pharmacopoeias, and is consumed primarily as flavored tea, as infusion or decoction. It is used in the symptomatic treatment of conditions related to the digestive and nervous systems (4), and in traditional medicine, to treat insomnia, anxiety, migraines, hypertension, as well as gastric, bronchial and psychiatric conditions. Its essential oil (Melissae aetheroleum) is an efficient antimicrobial agent, effective against gram-positive bacteria, as well as herpes viruses (16, 28); so, it is a promissory agent in front to the resistant bacterial strains.
However, the marketed material does not always correspond to the official species. Often it is adulterated and, or substituted with Nepeta cataria L., another aromatic Lamiaceae (37), a drug potentially toxic by the presence of an iridoid (nepetalactone) and their diastereoisomers in its essential oil (26), and therefore included in restrictive lists (2). Other few species can be known by similar common names, and may be involved in adulterations at local level due to misidentifications in the field during the harvest, or confused by poorly trained workers. Also the essential oil of M. officinalis is subject to tampering or replacement due to its high cost (17).
A number of phytochemical and pharmacological researches were carried out on these species, particularly on M. officinalis, a perennial and aromatic herb, whose dried leaves showed digestive, carminative, antiemetic, antispasmodic, sedative, anxiolytic and even antidepressant properties, among others, while its essential oil has antimicrobial, antifungal and antispasmodic actions (4, 6, 8, 29, 34). Meanwhile, N. cataria also has applications predominantly in digestive disorders and nervous system affections, being used as a carminative, digestive and antispasmodic, and as an anxiolytic, sedative and antidepressant, even antihysteric, hypnotic and antiodontalgic (8, 15). However, very few studies have been developed to determine the quality of the herbs and herbal formulations in these species (3), including comparative mineral content (24).
On the other hand, M. officinalis stands out as an important potential source of rosmarinic acid (an ester of caffeic acid), along with other species of the same family especially Mentha spicata L. (18, 25, 30), and even materials from hairy roots cultures of different Lamiaceae (12).
The aim of this study is to provide additional evidence (quantitative micrograph parameters, and HPLC and FTIR spectra) to identify fully the genuineness of the official drug, their mixtures and extracts by means of a number of diacritical characters.
MATERIALS AND METHODS
Materials
Fresh samples were obtained from three Argentinean populations of each adventitious (N. cataria) and cultivated (M. officinalis) plant species. A portion of the material was intended for taxonomic documentation (voucher specimens):
a) Melissa officinalis: L. A. Del Vitto & E. M. Petenatti #9246 (UNSL).
b) Nepeta cataria: L. A. Del Vitto, E. M. Petenatti & M. E. Petenatti #7437 (UNSL).
The material devoted to morphoanatomical studies were fixed and preserved in FAA (formalin: acetic acid: alcohol). Undoubtedly identified material from Herbarium UNSL served as secondary standards. Additionally were analyzed 37 samples of medicinal herbs labeled "Melissa" and "Toronjil", purchased in health food stores, pharmacies and popular markets of the region, whose vouchers are preserved in the Herbal Section of the UNSL Herbarium, National University of San Luis.
Methods
The species were classified according to the classical taxonomic methodology. Semi-permanent preparations were obtained by freehand cut, coloration with iodine green-carmine alum and mounting in glycerin jelly (7). Fresh leaves for quantitative micrograph techniques were diaphanized (9) and stained with 1% safranin, determining the following parameters (10): stomatal number (SN), stomatal index (SI), palisade ratio (PR), vein-islet number (VIN) and veinlet termination number (VTN). SN was measured with a 40x objective and the other parameters with 20x objective. The market samples were hydrated with hot water and detergent and then treated in the same way as those fresh. Both histological preparations and secondary standards of plant drugs were deposited in the Herbarium of the Universidad Nacional de San Luis (UNSL).
Macro- and micro-morphological observations were made using a stereomicroscope Leica M-10® (Leica Microsystems GmbH, Germany), and an optical microscope DMRB® (Leitz-Wetzlar, Germany), respectively; the photomicrographs were obtained with a digital camera EC-3® (Leica Microsystems GmbH, Germany) connected to the image capture system LAS EZ® v software 1.7.1 (Leica Microsystems Ltd., Germany).
Aerial plant parts (3 batches per each species) were harvested and dried in the shade until hygroscopic moisture; later were grinded using a mill Wiley® 3379 (Thomas Wiley, USA) with a stainless steel container, and a sieve up to 0.50 mm diameter. To prepare methanolic extracts (ME) and aqueous extracts (AE), 5g from the three batches of each species were used. ME were obtained extracting the drug with cold methanol until color exhaustion (3 per 24 hours) and then evaporated under vacuum using a rotatory evaporator Rotavapor® R-210 (Büchi Labortechnik AG, Switzerland). AE were obtained according the Argentinean Pharmacopoeia (11), soaking 5g of crude drug in 100mL of boiling distilled water, covering the container for 5 min and removing the solid by filtration; the aqueous portion was lyophilized with manifold type drum LT-16 (Rificor®, Argentina).
HPLC profiles were obtained from 1g of each extract, eluted with water: methanol (50:50; v/v) up to 100mL. Ten aliquots (20μL) for each batch were injected into an autosampler HPLC UltiMate® 3000 (Dionex, USA) with a diode array detector (DAD) and a Gemini® C18 (Phenomenex, USA) column (250 x 4mm i.d.; 5μm) termostated at 25°C, using the mobile phase water: methanol (50:50; v/v) run at a flow rate of 0.5mL min-1 during 30min.; the UV spectra were recorded in the range of 200-367nm. The chromatograms obtained for each species at 320 nm were compared with those of a standard sample of ≥98% rosmarinic acid (Sigma Aldrich®); the data were processed with a software Chromeleon® (Thermo Fisher Sci., USA).
To accomplish FTIR spectra, 12 batches per each population of the two species were pelletized with KBr, making a dispersion of each solid sample (lyophilized EA or EM) in KBr (3:100 w/w) and homogenized in mortar and pestle. About 100 mg of the homogenized mixture was placed between two metal plates in the cylinder of a hydraulic press, then applying a pressure of 500 kg cm-2; allowed pressed 5 min., vented and placed the tablet into a sample holder, then obtaining the spectra in a spectrophotometer Protégé® 460 (Nicolet), equipped with a CsI beamsplitter. Values were obtained with a spectral resolution of 4 cm-1 in the range between 4000 and 460 cm-1.
All reagents used were of analytical and / or HPLC grade.
The data collected were subjected to statistical analysis (one-way ANOVA and the instrumental own statistical programs).
RESULTS AND DISCUSSION
Melissa officinalis L. subsp. officinalis was described in 1753 (Linnaeus, Species plantarum 2: 592) and belongs to the Family Lamiaceae (nom. alt. Labiatae), Subfam. Nepetoideae, Tribe Mentheae. The pharmacopoeial approved name for dry leaves and herb of this species is Melissae folium (34). The vulgar names are "melisa", "toronjil" (Spanish), "erva cidreira" (Portuguese); "balm", "lemon balm" (English). For their part, Nepeta cataria L. was described at the same time than the former (Linnaeus, Species plantarum 2: 570, 1753) and belongs to the same tribe Mentheae; their vulgar names are "nepeta", "hierba gatera", "menta de los gatos", "nébeda" or "toronjil" (Spanish), "erva-gateira" (Portuguese), "catmint", "catnip", or "catnep" (English).
Quantitative Micrograph and Leaf Anatomical Characters
The leaves of M. officinalis show a thin, smooth cuticle, except in the abaxial surface on the nerves, that is finely striated. Both epidermis are unistrate with sinuate radial cell walls. The blade is hypostomatic (stomata present only on the abaxial epidermis), with raised diacytic stomata, the number of stomata is 17.71 ± 4.62, and the stomatal index had values among 14.58 to 22.33. The palisade ratio ranged from 6.54 to 12.82. The nerves are prominent on the abaxial surface.
The indument consists of four types of trichomes: two simple non-glandular, some of them very short, 1-celled, conical, straight to clawed, and other long, 2-5-celled, uniseriate, wide base and acute apex, with warty walls (around the nerves and margin are 6-7-celled), with higher density in the back; and two types of glandular trichomes, some of them small, capitates, with 1- or 2-celled foot and 1-2-celled head, primarily present in the upper epidermis, and others large, peltate, short-footed, 1-celled, and spheroid or ovoid 8-celled head (the characteristic type of secretory trichome in Lamiaceae), present in depressions in the lower epidermis.
The trichomatic typology agrees with that reported for Lamiaceae by various authors and in particular to this species by Padurariu et al. (2009). The mesophyll is dorsiventral with unistrate palisade parenchyma to the upper surface, with 3-4 cell layers of spongy parenchyma with small meatus to the lower surface. The collateral vascular bundles are coated by a non Kranz parenchymatic sheath that consists of several cell layers. Vein-islet number was 5.25 ± 1.48, and the veinlet terminations number was 1.57 ± 1.06.
Meanwhile, a cross-section of the leaf of N. cataria show a dorsiventral structure with similar features to M. officinalis, differing particularly in the indument, which is formed here by highly abundant simple non-glandular 3-5 celled trichomes, with very warty walls, and only one type of glandular trichome, with 2-celled foot and 1-celled head, lacking peltate trichomes that characterize Melissa officinalis. The blade is also hypostomatic, with raised diacytic stomata, the stomatal number is 16.21 ± 3.26, and stomatal index had values among 17.11 to 20.21. The palisade ratio ranged from 2.93 to 3.98.
The mesophyll present a unistrate palisade parenchyma towards upper surface, while 3-4 cell layers spongy parenchyma occurs toward the lower surface. The vascular bundles, collateral, are covered by a non Kranz sheath parenchyma, and protected by angular collenchyma. Vein-islet number was 5.87 ± 1.05, and the veinlet termination number was 3.13 ± 1.25. A summary of the quantitative micrographic parameters is shown in table 1 (page 20).
Table 1. Quantitative micrographic parameters of M. officinalis and N. cataria.
Tabla 1. Parámetros micrográficos cuantitativos de M. officinalis y N. cataria.

Both species show hypostomatic leaves, and therefore only have stomata in abaxial surface. Specific differences about stomatal number were not significant (p<0.05), while the stomatal index corresponding to M. officinalis varies widely (14 to 22), which fully includes the narrower dispersion data that shows N. cataria (17 to 20). Palisade ratio of M. officinalis ranges from about 6 to 13, a relatively high and significant value (p<0.05) compared to that of N. cataria (3 to 4). Vein islet number of both species was very similar, while the differences found in the veinlet termination number were significant (p<0.05), because in N. cataria this parameter is double that of M. officinalis. In summary, the most useful parameters to distinguish these drugs are palisade ratio (PR) and veinlet termination number (VTN).
High Performance Liquid Chromatography (HPLC)
Applying HPLC techniques to samples of both species, as well as a standard of rosmarinic acid, resulted in the curves of figure 1, figure 2 (page 21) and the values shown in table 2 (page 21).
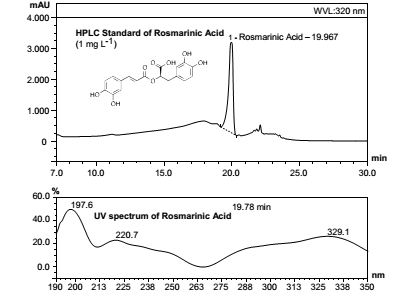
Figure 1. HPLC profile and UV spectrum from a standard of rosmarinic acid (1 mg L-1).
Figura 1. Perfil de HPLC y espectro UV de un patrón de ácido rosmarínico (1 mg L-1).

Figure 2. HPLC profiles from samples of M. officinalis and N. cataria.
Figura 2. Perfiles de HPLC de ejemplares de M. officinalis y N. cataria.
Table 2. Rosmarinic acid average concentration of genuine crude drug samples of Melissa officinalis and Nepeta cataria, by HPLC (n=3 for each population, 9 in all for each species).
Tabla 2. Concentración promedio de ácido rosmarínico en ejemplares genuinos de droga cruda de M. officinalis y N. cataria, obtenida mediante HPLC (n=3 para cada población, 9 en total para cada especie).

According to these results, analyzed raw drugs corresponding to M. officinalis, which are cultivated, and marketed or use in Argentina, containing about 35 to 43 g Kg-1 of rosmarinic acid expressed in a dry weight basis, a proportion 15 to 21 times higher than those corresponding to N. cataria adventitious or marketed in the region as substitutes for M. officinalis (2 to 2.4 g Kg-1 dry weight). The values obtained here were similar to those reported for various sources of both drugs (5, 20).
As the populations of N. cataria which are adventitious and exceptionally cultivated in Argentina, the low concentration of rosmarinic acid, together with its nepetalactone content, we can affirm that this plant does not participate fully in the properties attributed and / or checked for M. officinalis, thereby constituting an adulterant, rather than a suitable commercial substitute.
Meanwhile, it has been reported that a variety of N. cataria known as "lemon catnip" (N. cataria L. var. citriodora (Dumort.) Lej.), lacks nepetalactone, unlike other species and varieties of the genus, instead containing between 0.6 to 1.5 mg Kg-1 of rosmarinic acid as well as other phenolic acids, monoterpenes (nerol, geraniol, and citral), and flavonoids (21). Although from a taxonomic point of view this variety is no longer valid, being within the broad concept of the N. cataria species, could be considered a chemical variety because distinctive chemical composition. So, this would be the only taxon closely related to N. cataria which could replace M. officinalis, for the purpose of using these drugs in infusion, because their potential action as a sedative, antispasmodic, anti-inflammatory and antioxidative.
Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra, that have been successfully used in the of characterization and / or determination of authenticity in various species and / or chemotypes of medicinal drugs and other plant samples (1, 14, 19, 22, 26), were obtained in this case from each of the extracts of M. officinalis and N. cataria, determining the areas under the curves of specific vibrational modes, in this case corresponding to the aliphatic high frequency CH and CO stretching modes, one of the tools that can be used to study possible differences among medicinal plants, obtaining the results shown in figures 3-4 and table 3 (page 23); table 4 (page 25).

Figure 3. FTIR spectra of M. officinalis. Methanolic extract (A) and lyophilized aqueous extract (B).
Figura 3. Espectros FTIR de M. officinalis. Extractos metanólico (A) y acuoso liofilizado (B).
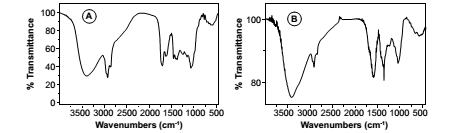
Figure 4. FTIR spectra of N. cataria. Methanolic extract (A) and lyophilized aqueous extract (B).
Figura 4. Espectros FTIR de N. cataria. Extractos metanólico (A) y acuoso liofilizado (B).
Values obtained have allowed establishing the comparative table 3.
Table 3. Area values and their relationship, obtained from FTIR in methanolic and dried aqueous extracts of M. officinalis and N. cataria (n=12 for each population, 36 in all for each species).
Tabla 3. Valores de área y sus interrelaciones, obtenidos por FTIR en extractos metanólico y acuoso liofilizado de M. officinalis y N. cataria (n=12 para cada población, 36 en total para cada especie).
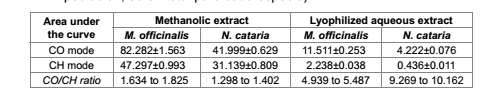
Table 4. Main diacritical features that allow characterize Melissa officinalis and Nepeta cataria drugs.
Tabla 4. Principales caracteres diacríticos que permiten identificar las drogas Melissa officinalis y Nepeta cataria.

The spectra show high frequency bands in the area of OH and CH vibration modes which differ in 25 cm-1. At lower frequencies, in the region corresponding to the CO stretching, major differences were observed. The areas under the curves of the CH and CO modes can be used to find interspecific differences.
Indeed, considering the methanolic extract obtained from both species, the areas under the curve from M. officinalis (figure 3a, page 22; table 3), showed mean values of 82.282 for the mode CO, and 47.297 for the mode CH, whereby the ratio CO/CH varied from 1.634 to 1.825. In turn, N. cataria showed average areal values of 41.999 for the mode CO, and 31.139 for the mode CH, and a CO/CH rate from 1.298 to 1.402.
Although the aqueous extracts also showed different vibrational patterns, is their area ratio which allows to differentiate the two species. The areas for the lyophilized aqueous extract of M. officinalis (figure 3b, page 22; table 3) showed values of 2.238 for CH modes and 11.511 to CO modes, and in this case the CO/CH ratio varied from 5.939 to 5.487, while the areas recorded for the correspondent extract from N. cataria (figure 4b, page 22; table 3) reached 4.222 for CO mode and 0.436 for CH mode, and exhibited a CO/CH ratio from 9.269 to 10.162.
For both species, there were differences in the CO / CH ratio, which were significant in reference to the methanolic extract, while lyophilized extracts showed highly significant differences among the two species. This is likely to be directly related to increased water solubility of the compounds present in these drugs.
Although these two species share a number of organic compounds (α-humulene, β-elemene, caryophyllene-oxide, cis-ocimene, citral, citronellal, geraniol, myrcene, nerol, rosmarinic acid, thymol, and trans-ocimene), they are more numerous differentials secondary metabolites (mostly essential oils) that have been reported to date in M. officinalis (79 compounds) than in N. cataria (32 compounds) (31). These differences may explain the diverse vibrational stretching values obtained for extracts of both species.
Therefore, it can be argued that, even in the case of multi-molecular complexes like this, these specific differences have semi-quantitative relevance. Thus, IR spectroscopy may be a useful tool for the characterization of M. officinalis and N. cataria, demonstrating the ability of the method to find additional characters to those obtained by other pharmacognostic methods. Moreover, and far as we know, this is the first mention of FTIR data (especially on CO/CH ratio) applied to the identification of specimens of these two medicinal species.
CONCLUSIONS
The herbs marketed and/or used in southern South America under the names "Melissa" or "Toronjil" do not always match with the official species, Melissa officinalis (even under its pharmacopoeial name: Melissae folium).
The adulterant (or partial substituent) more frequently found in M. officinalis commercial samples is Nepeta cataria, another Lamiaceae, whose presence is due to the morphological affinity, an erroneous harvest, and/or their sometimes coincident common names.
Adventitious/cultivated populations of N. cataria or marketed drug from this species in Argentina are chemotypes rich in the iridoid nepetalactone. Therefore, the adulteration of M. officinalis with N. cataria drug used in Argentina can become a high health risk.
Only the N. cataria chemotype known as "lemon catnip" (N. cataria var. citriodora), lacks nepetalactone, and because its chemical composition could represent a substituent for M. officinalis.
At micrographic level, we report for the first time comprehensive quantitative micrographic parameters of M. officinalis and N. cataria, far as we know. The decisive quantitative parameters to identify each of these drugs are the palisade ratio and the veinlet termination number per mm2.
The HPLC profiles of both species allow their differentiation through the high content of rosmarinic acid in M. officinalis, in comparison to the low amount that shows N. cataria.
FTIR spectra of both species are reported for the first time, for what we know. Even with the limitations of a semiquantitative method, these spectra also contribute to the differentiation among the species, both the vibrational modes as areal values obtained for each in the fingerprint zone.
These contributions to the recognition of both species can facilitate the pharmacognostic quality control both crude drugs (even when the material is finely ground or reduced to powder) and the extracts used in the pharmaceutical industry. An overview of drug diacritical characters between the two species sold in Argentina is displayed in table 4 (page 25).
1. Adiana, M. A. ; Masura, M. P. 2011. Study on Senna alata and its different extracts by Fourier transform infrared spectroscopy and two-dimensional correlation infrared spectroscopy. J Mol Structure. 991: 84-91. [ Links ]
2. ANMAT (Administración Nacional de Medicamentos, Alimentos y Tenología Médica). 2009. Anexo VI, Listado negativo de plantas tóxicas. In: http://www.anmat.gov.ar/ webanmat/mercosur/ pdf_files/01ag_coprosal/AGREGADO_VIII_MODIFICACION_FITOTERAPICOS%20arg.pdf. Retrieved October 15, 2013 [ Links ]
3. Arceusz, A.; Weselowski, M. 2013. Quality consistency evaluation of Melissa officinalis L. commercial herbs by HPLC fingerprint and quantitation of selected phenolic acids. J Pharm & Biomed Analysis. 83: 215-220. [ Links ]
4. Blumenthal, M.; Goldberg, A.; Brinckmann, J. 2000. Herbal medicine, Expanded Commission E monographs. Newton (MA, U.S.A.), Integrative Medicine Communications. [ Links ]
5. Carnat, A. P.; Carnat, A.; Fraisse, D. ; Lamaison, J. L. 1998. The aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. subsp. officinalis) tea. Pharm Acta Helv. 72: 301-305. [ Links ]
6. Coleta, M.; Campos, M. G.; Cotrim, M. D.; da Cunha, P. 2001. Comparative evaluation of Melissa officinalis L., Tilia europaea L., Passiflora edulis Sims. and Hypericum perforatum L. in the elevated plus maze anxiety test. Pharmacopsychiatry. 34 (suppl. 1): S20-1. [ Links ]
7. D'Ambrogio, A. 1986. Manual de técnicas en histología vegetal. Buenos Aires, Hemisferio Sur. [ Links ]
8. Del Vitto, L. A.; Petenatti, E. M.; Petenatti, M. E. 1998. Recursos herbolarios de San Luis (Argentina). Segunda parte: plantas exóticas cultivadas, adventicias y/o naturalizadas. Multequina. 7: 29-48. [ Links ]
9. Dizeo de Strittmater, C. 1973. Nueva técnica de diafanización. Bol Soc Argent Bot. 15: 126-129. [ Links ]
10. Evans, W. C. 2002. Trease & Evans Pharmacognosy, 15th ed. Edimburg: W. B. Saunders. [ Links ]
11. Farmacopea Argentina, 1978. Codex medicamentarius argentino. 6th ed. Buenos Aires, Comisión Permanente de la Farmacopea Argentina. [ Links ]
12. Fattahi, M.; Nazeri, V.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R. M.; Zamani, Z.; Palazon, J. 2013. A new biotechnological source of rosmarinic acid and surface flavonoids: Hairy root cultures of Dracocephalum kotschyi Boiss. Industrial Crops & Products. 50: 256-263. [ Links ]
13. Fugh-Berman, A. 2000. Herb-drug interactions. The Lancet. 355(9188): 134-138. [ Links ]
14. Gad, H. A.; El-Ahmady, S. H.; Abou-Shoerb, M. I.; Al-Azizia, M. M. 2013. Application of Chemometrics in Authentication of Herbal Medicines: A Review. Phytochem Anal. 24: 1-24. [ Links ]
15. Gilani, A. H.; Shah, A. J.; Zubair, A.; Khalid, S.; Kiani, J.; Ahmed, A.; Rasheed, M.; Ahmad, V. U. 2009. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J Ethnopharmacol.121(3): 405-411. [ Links ]
16. Hancianu, M.; Aprotosoaie, A. C.; Gille, E.; Poiata, A.; Tuchilus, C.; Spac, A.; Stanescu, U. 2008. Chemical composition and in vitro antimicrobial activity of essential oil of Melissa officinalis L. from Romania. Rev Med Chir Soc Med Nat Iasi. 112(3): 843-847. [ Links ]
17. Hener, U.; Faulhaber, S.; Kreis, P.; Mosandl, A. 1995. On the authenticity evaluation of balm oil (Melissa officinalis L.). Pharmazie. 50(1): 60-62. [ Links ]
18. Janicsák, G.; Máthé, I.; Miklóssy-Vári, V.; Blunden, G. 1999. Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochem Syst & Ecol. 27(7): 733-738. [ Links ]
19. Konwar, M.; Baruah, G. D. 2011. On the nature of vibrational bands in the FTIR spectra of medicinal plant leaves. Archiv Appl Sci Res. 3(1): 214-221. [ Links ]
20. Lamaison, J. L.; Petitjean-Freytet, C.; Duband, F.; Carnat, A. P. 1991. Rosmarinic acid content and antioxidant activity of French Lamiaceae. Fitoterapia. 62: 166-171. [ Links ]
21. Modnicki, D.; Tokar, M.; Klimek, B. 2007. Flavonoids and phenolic acids of Nepeta cataria L. var. citriodora (Becker) Balb. (Lamiaceae). Acta Pol Pharm, Drug Res. 64(3): 247-252. [ Links ]
22. Muruganantham, S.; Anbalagan, A.; Ramamurthy, N. 2009. FT-IR and SEM-EDS comparative analysis of medicinal plants, Eclipta alba Hassk. and Eclipta prostrata Linn. Roman J Biophys. 19(4): 285-294. [ Links ]
23. Padurariu, C.; Gales, R.; Preotu, A.; Zamfirache, M. M.; Toma, C.; Boz, I. 2009. Distribution and morphology of Melissa officinalis L. vegetative organs. Analele stiintifice ale universitatii "Al. I. Cuza" Iasi 55(2), Biologie Vegetala: 21-25. [ Links ]
24. Petenatti, M. E.; Petenatti, E. M.; Del Vitto, L. A.; Téves, M. R.; Caffini, N. O.; Marchevsky, E. J.; Pellerano, R. G. 2011. Evaluation of macro and microminerals in crude drugs and infusions of five herbs widely used as sedatives. Braz J Pharmacogn. 21(6): 1144-1149. [ Links ]
25. Petersen, M.; Simmonds, M. S. J. 2003. Rosmarinic acid. Phytochemistry. 62(2): 121-125. [ Links ]
26. Rui, J. L.; Sun, S. Q.; Wang, X. X.; Xu, C. H.; Chen, J. B.; Qun, Z.; Lu, G. H. 2014. Differentiation of five species os danggui raw material by FTIR combined with 2D-COS IR. J Mol Structure. 1069: 229-235. [ Links ]
27. Sastry, S.; Springstube, W.; Waller, G. 1972. Identification of 5,9-dehydronepetalactone, a new monoterpene from Nepeta cataria. Phytochemistry. 11(1): 453-455. [ Links ]
28. Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. 2008. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine. 15(9): 734-740. [ Links ]
29. Schulz, V.; Hansel, R.; Tyler, V. E. 1998. Rational Phytotherapy, A Physicians' Guide to Herbal Medicine. 3rd ed. Berlin, Springer. [ Links ]
30. Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A. R.; Hamedani, M. P. 2012. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacognosy Mag. 8(29): 37-41. [ Links ]
31. USDA/ARS/GRIN, 2013. Dr. Duke's Phytochemical and Ethnobotanical Databases. In: www.ars-grin. gov/duke/. Retrieved October 15, 2013. [ Links ]
32. Vercelli, N.; Entraigas, I.; Scaramuzzino, R.; Migueltorena, V.; D' Alfonso, C. 2013. Plantas medicinales de los bajos alcalinos de la cuenca del arroyo del Azul (provincia de Buenos Aires, Argentina). Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 45(2): 285-298. [ Links ]
33. WHO (World Health Organization), 1998. Quality control methods for medicinal plant materials. Geneva, WHO. [ Links ]
34. WHO (World Health Organization), 2002. WHO monographs on selected medicinal plants. Vol. 2. Geneva, WHO. [ Links ]
35. WHO (World Health Organization), 2004. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva, WHO. [ Links ]
36. WHO (World Health Organization), 2007. Expert Committee on Specifications for Pharmaceutical Preparations, 41st rep. Geneva, WHO Tech. Rep. Series 943. [ Links ]
37. Zeichen, R.; Gargiulo, S.; Carena, M.; Bindstein, E. 2004. Estudio farmacológico comparativo de dos especies argentinas: Nepeta cataria L. (Labiatae) y Melissa officinalis L. (Labiatae). BLACPMA. 3(6): 103-106. [ Links ]
Acknowledgements
We express our gratitude to the SPU-ME 22Q/416 and SECyT-UNSL 4-8702 Projects for their financial support, the Editorial Board of the Journal and the anonymous referees for their valuable suggestions.














