Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
Print version ISSN 1853-8665On-line version ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.51 no.1 Mendoza June 2019
ORIGINAL ARTICLE
Effect of chitosan coating enriched with oregano essential oil on the quality of refrigerated meat hamburgers
Efecto de un recubrimiento de quitosano con aceite esencial de orégano en la calidad de hamburguesas vacunas refrigeradas
Claudia Amadio 1, Silvina Farrando 2, Mónica Zimmermann 1
1 Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias. Cátedra de Industrias Agrarias. Alte. Brown 500. Chacras de Coria. Mendoza. Argentina. M5528AHB. camadio@fca.uncu.edu.ar
2 Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias. Cátedra de Microbiologia Agrícola e Industrial. Alte. Brown 500. Chacras de Coria. Mendoza. Argentina. M5528AHB.
Originales: Recepción: 04/08/2017 - Aceptación: 01/06/2018
ABSTRACT
Antimicrobial and antioxidant properties of oregano essential oil (OEO) have been extensively reviewed. Its application may adversely impact the sensory perception of food. In this sense, an interesting approach to reduce its dose, while maintaining effectiveness, could be to incorporate these compounds into coatings. To determine the antioxidant and antimicrobial potential of chitosan coatings with OEO in hamburgers stored at 4°C, 3 batches were elaborated with 1% v/v acetic acid in water (control), pure chitosan (1%), chitosan with addition of 2% OEO. Stability was assessed by radical-scavenging activity (DPPH), thiobarbituric acid reactive substances (TBARS), pH, color, microbiological analysis and sensory evaluation (hedonic scale of 5 points). The addition of OEO to the coating improved the antioxidant property and the red color (a*) of these burgers was always higher. The pH was not significantly affected by treatments. All samples showed similar yeast and fungal counts, while the psychrophilic bacteria increased with the addition of OEO to the coating. Sensorially, the treatment with chitosan + OEO coating obtained the highest score in color and smell, along with the control. Taste and acceptability deteriorated over time. The addition of OEO to the coating of chitosan allowed improving the antioxidant property of chitosan and therefore the color of the hamburgers, while sensorially accepted. There was no evidence of a preservative effect.
Keywords: Chitosan; Conservation; Oregano essential oil; Hamburger; Edible coatings; Food quality
RESUMEN
Las propiedades antimicrobianas y antioxidantes del aceite esencial de orégano (AEO) han sido ampliamente revisadas. Su aplicación en los alimentos puede tener un impacto adverso en la percepción sensorial. En este sentido, un enfoque interesante para reducir su dosis, manteniendo su efectividad, podría ser incorporar estos compuestos en los recubrimientos. Para determinar el potencial antioxidante y antimicrobiano de recubrimientos de quitosano con aceite esencial de orégano (AEO) en hamburguesas almacenadas a 4°C, se elaboraron 3 lotes que fueron formulados con: ácido acético al 1% v/v en agua (control), quitosano puro (1%), quitosano adicionado de 2% de AEO. La estabilidad se valoró mediante capacidad de secuestro de radicales libres (DPPH), sustancias reactivas al ácido tiobarbitúrico (TBARS), pH, color, análisis microbiológico y evaluación sensorial (escala hedónica de 5 puntos). La adición de AEO al recubrimiento, mejoró la propiedad antioxidante y el color rojo (a*) de estas hamburguesas fue siempre superior. El pH no fue afectado significativamente por los tratamientos. Todas las muestras presentaron similares recuentos de levaduras y hongos, mientras que las bacterias psicrófilas aumentaron con el agregado de AEO al recubrimiento. Sensorialmente, el tratamiento con recubrimiento de quitosano+AEO obtuvo el mayor puntaje en color y en olor junto con el control. El sabor y la aceptabilidad se deterioraron a través del tiempo. El agregado de AEO al recubrimiento de quitosano permitió mejorar la propiedad antioxidante del quitosano y por lo tanto el color de las hamburguesas, siendo aceptadas sensorialmente. No se evidenció efecto conservante.
Palabras clave: Quitosano; Conservación; Aceite esencial de orégano; Hamburguesa; Recubrimiento comestible; Calidad de alimentos
INTRODUCTION
Meat and especially ground meat products, are rich in protein, lipids and have suitable moisture content, which make them highly susceptible to microbial growth. Meat is also susceptible to lipid oxidation that can deteriorate its sensorial properties, by generation of compounds such as aldehydes, ketones, alcohols, acids, and hydrocarbons which are associated with a rancid taste and odor (36). Lipid oxidation in red meat also results in color losses. Ground beef is a significant part of meat products, particularly when used for hamburger production. When the beef meat is grounded to form the burger, the integrity of the muscle membrane is broken, increasing the surface area that promotes lipid oxidation and microbial growth of this stored meat products (23).
The potential source of contamination depends on the condition of the animal before, during and after slaughter and the transportation, marketing and consumer handling of the meat. Microorganisms such as Pseudomonas spp., and Enterobacteriaceae can cause spoilage (29). Contamination can also be caused by psychrotrophic and pathogenic species such as Staphylococcus aureus, Listeria monocytogenes, Clostridium perfringens, Campylobacter jejuni and Yersinia enterocolitica and by enteropathogenic bacteria such as Escherichia coli and Salmonella spp. (15). Consequently, controlling microbial growth and lipid peroxidation in beef patties is a crucial strategy for sustaining the safety, nutritional and sensory potential of these products.
Although synthetic additives have been widely used in the meat industry to inhibit both process of lipid oxidation and microbial growth, natural additives are preferred in place of them in recent years because of the growing concern among consumers about such chemical additives (29).
An edible coating is a thin layer of edible material formed as a coating on a food product, used to mechanically protect food, prevent the contamination from microorganisms and prevent quality loss of food due to mass transfer (e.g. moisture, gases, flavours, etc.).
In addition, coatings by themselves or acting as carriers of food additives (i.e.: antioxidants, antimicrobials), have been particularly considered in food preservation, given their ability to extend the shelf life. Many different substances have suitable properties for coating use, namely polysaccharides, lipids and proteinbased polymers. They can be applied by different methods: with a paint brush or by spraying, dipping-dripping, fluidizing, etc. Coating is the most commonly used method for fruits, vegetables and meat products. Chitosan, a fibre of animal origin (i.e.: exoskeleton of crustaceans, insects), is one of such substances. Chitosan is a polysaccharide obtained by deacetylation chitin with the advantage of being considered a biobased environmentally friendly material (9). Chitosan has been reported to have antimicrobial activity against bacteria, fungi and yeasts (2), and antioxidant properties measured by DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging activity, hydroxyl radicals scavenging, ferrous ion chelating ability tests and 2-thiobarbituric acid reactive substances (TBARS) test (21).
Origanum vulgare (Lamiaceae) is a perennial herb distributed in Europe, North Africa, America and Asia. This herb have wide culinary uses and industrial applications due to the high antioxidant and antimicrobial activity of their main essential oil compounds, thymol and carvacrol.
The ability of plant essential oils (EOs) to protect food against pathogenic and spoilage microorganisms and oxidation, have been reported by several researchers (4, 5). Nevertheless, the amount of essential oils used in order to achieve effective antimicrobial and antioxidant activity is determinant for the sensory acceptance, as strong aromas of essential oils might be imparted to food products (12). Therefore, new research should focus on incorporation of essential oils to edible coatings as a supplementary application in food packaging (10).
It has been shown that chitosan films enriched with oregano essential oil, are an excellent system to control the release of active compounds (26). A combined application of chitosan and OEO, (containing two important active compounds thymol and carvacrol) was shown to have antioxidative and antimicrobial effects in different mediums (11).
To the best of knowledge, the application of chitosan as a single agent, or in combination with OEO, has not been studied to date in meat patties. Thus, the objective of the present work was to determine the effect of chitosan and OEO on microbiological, physicochemical and sensory parameters of meat patties.
MATERIALS AND METHODS
Natural antioxidants and chemicals
It was worked with the aerial parts of flowering plants of a monoclonal variety of oregano: Alpa Sumaj, grown in La Consulta, Mendoza (INTA Experimental Station: coordinates 33°73' S 69°12 W), Argentina.
The essential oil of oregano (OEO) was extracted by steam distillation process. The essential oils obtained were stored at 4°C until further tests.
Low molecular weight chitosan (LMW, 50-190 kDa, Degree of deacetylation; DD = 75-85%), in powder form, was obtained (Sigma-Aldrich. Co., Germany).
All reagents used were of analytical grade or the highest grade available and were obtained either from Sigma-(Sigma-Aldrich, USA) or Merck (Darmstadt, Germany).
Chitosan solutions and films preparation
Chitosan (1% or 2%, w/v) was dispersed in an aqueous solution of glacial acetic acid (1%, v/v). The solution in beakers was placed on a Decalab magnetic stirrer (Argentina) with heating. Glicerol was added to chitosan at a concentration of 0.75 ml/g as a plasticizer and stirred for 10 min. Then, the OEO, mixed with Tween 20, to help distribute and completely incorporate the oregano oil, was added to the chitosan solution. The chitosan solution was prepared without addition of OEO.
To prepare the films, 30 mL of each solution were cast onto 90 mm Petri dishes and placed in an oven at 20°C for 72 h; they were subsequently stored at 20°C and 54% RH, in a desiccator containing a saturated solution of Mg (NO3)6H2O (34), until further use.
Hamburger manufacture and treatment
Ground beef was purchased from a local grocery store and stored at 4°C before patty preparation. The meat was blended by hand and was moulded in Petri dishes (6 cm diameter) to obtain the hamburgers.
The beef patties were prepared all with 1% (w/w) added NaCl, and coating using a sponge brush, with the following solutions: aqueous solution of glacial acetic acid (1%, v/v) (control), chitosan solution (Ch), chitosan and 2% OEO (Ch+2%OEO). Afterwards, they were dried at room temperature under natural convection for 2-3 h, separately wrapped with polyvinyl chloride (PVC) films and stored in temperature-controlled laboratory refrigerator (4°C) for up to 9 days. Sampling and storage conditions records from each treatment took place at 0, 3, 6 and 9 days.
DPPH radical scavenging activity
The free radical scavenging activity of chitosan film was measured by 1,1-diphenyl-2-picryl-hydrazil (DPPH•) using the method of Byun et al. (2010) with a slight modification. Film (0.1 g) was mixed with 2 mL of ethanol for 3 min and allowed to stand at room temperature for 3 h. Then, it was vigorously vortexed for another 3 min and centrifuged at 2300 rpm for 10 min. An aliquot of ethanol extract (3 mL) was mixed with 10 mL of 0.1 mM DPPH in ethanol.
The absorbance at 517 nm was measured after the solution had been allowed to stand in the dark at room temperature for 30 min.
The radical scavenging activity (RSA) of the chitosan films was calculated according to the equation:
RSA (%)= ((Absorbanceblank-Absorbance sample)/ Absorbanceblank))×100
where:
Absorbance sample = represents the absorbance of the sample solution
Absorbance blanck= represents the absorbance of DPPH solution without the addition of the film
Agar diffusion method
The sensitivity of Listeria, Escherichia coli, Staphylococcus aureus, Salmonella, Bacillus cereus, Lactobacillus, E. coli O157:H7, Pseudomonas and Campylobacter to the chitosan solution, was determined by agar diffusion methods. For experimental use, an overnight culture was adjusted by comparison against McFarland 0.5 standard 1-5 x 106 CFU (colony forming units) mL-1. 100 µL were inoculated by spreading to plates on Mueller Hinton Agar, in triplicate. 20 µL of extracts were poured into 4-mm agar-well. All inoculated plates were incubated at 30°C for 24 hours. After incubation, diameter of the inhibition zone was measured by using calipers.
The sensitivity to the extracts was classified by the diameter of the inhibition halos as: not sensitive (-), diameters less than 8 mm (+); sensitive, diameters 9 -14 mm (++); very sensitive, diameters 15-19 mm (++); and extremely sensitive, diameters larger than 20 mm (+++) (34).
Lipid oxidation measurement
The thiobarbituric acid reactive substances (TBARS) assay was performed as described by Buege and Aust (1978). 1.0 g beef paty was mixed with 3.0 ml of stock solution containing 0.375% (w/v) thiobarbituric acid, 15% (w/v) trichloroacetic acid, and 0.25 N HCl.
The mixture was heated for 10 min in a boiling water bath to develop a pink color, cooled in tap water and then centrifuged (4,300 g for 10 min).
The absorbance of the supernatant was measured spectrophotometrically at 532 nm. The amount of TBARS was expressed as milligrams of malondialdehyde per kilogram of sample.
pH values
The measurement of pH was carried out on 10 g of sample homogenized in distilled water (1:5 sample/water). The pH value of the sample was determined using a Sper Scientific 850081 pH meter (Sper Scientific Ltd, Scottsdale, AZ).
Color values
The colour coordinates were determined employing CIELab scale: lightness (L*), redness (a*, +/- red/green), and yellowness (b*, +/- yellow/blue). Colour determinations were made at 12 ± 2°C by means of a Minolta CR-400 (Minolta Camera Co., Osaka, Japan) Chroma Meter with illuminant D 65, 10° observer, 11 mm aperture of the instrument for illumination and 8 mm for measurement. An average value from eight random locations on each sample surface was used for statistical analysis.
In addition, hue angles (H) were calculated as follows: H = arctg (b*/a*).
Microbiological analyses
A 25 g portion from each treatment were aseptically added a 225 mL of 0.1% peptone water, homogenized and appropriate serial decimal dilutions were prepared in the same diluent.
Psychotropic heterotrophic bacteria (PB), total coliform bacteria (CB), thermotolerant coliform bacteria (TCB) and yeast and mould (YM) were tested according to ICMSF, 2000. PB were enumerated using plate count agar medium by the plate method and the plate incubated for 48 h at 15°C. CB were enumerated using the Most Probable Number technique (MPN) and series of 3 tubes per dilution.
Corresponding dilutions were inoculated in Mac Conkey Broth, with Durham bell. It was incubated at 35° C for 48 h. Tubes showing gas production were recorded as positive. Results were then interpreted by using tables of most probable number (MPN), and the index of coliforms per gram of food sample was obtained.
Positive Mac Conkey broth tubes were sub-cultured to brilliant green bile (2%) broth tubes, each with a Durham bell. After incubation at 44.5°C for 24-48 h, tubes showing gas production were recorded as positive. MPN tables were used to obtain the index of thermotolerant coliform bacteria per gram of original food sample.
Detection of Escherichia coli was performed from the positive tubes of thermotolerant coliforms, isolated in Methylene Blue Eosin Agar and incubated for 24 to 48 h at 37°C.
For the identification of this microorganism, the typical colonies of E. coli were tested IMViC (Indol, Methyl Red, VogesProskauer and Citrate).
YM were enumerated using Mould and Yeast agar medium by the pour plate method, with plate incubation for 5 days at 28°C following inoculation.
Sensory evaluation
Six experienced panelists were chosen from the staff members of the Faculty of Agricultural Sciences, National University of Cuyo, Mendoza, Argentina. Panelists were selected according to their habits, their familiarity with the patties to be analyzed, their sensitivity and the ability to reproduce the evaluation made.
The color and odor evaluations were performed on the raw samples and their attributes were rated with five-point descriptive scales (1-very bad, 2-bad, 3-acceptable, 4-good, 5-very good). Later, patties were cooked on conventional electric oven. The temperature of the centre of patties reached approximately 75°C. Each panelist received one-quarter portions of patties that were coded and served warm on ceramic plates. Panelists were invited to clean and rinse the palate between samples with unsalted cracker and lemon juice. Cooked burgers were evaluated for odor, taste and overall acceptability. Odor following the same scale described for raw burgers, and the others using a five-point hedonic scale, where 5= like very much, and 1= dislike very much.
Statistical analysis
All measurements were done in triplicates except color, that was measured eight times. The data is presented as means ± SD. Data collected for antioxidant test DPPH was analyzed by one-way ANOVA according to treatment type (chitosan, chitosan+1% OEO, chitosan+2% OEO).
For shelf-life, two factors ANOVA, type burger coating (control, Ch, Ch + 2% OEO) and storage time (0, 3, 6 and 9 days), were applied for each parameter. Means were compared with Tukey's test.
The sensory analysis data was analysed by the Friedman test and the existence of significant differences was established at α=0.05 level.
RESULTS AND DISCUSSION
DPPH radical scavenging activity
The RSA of chitosan films with and without incorporated OEO was determined and it is presented in table 1.
Table 1. Radical scavenging activity of the chitosan films with different concentrations of OEO.
Tabla 1. Actividad de secuestro de radicales libres de películas de quitosano con distintas concentraciones de AEO.

DPPH tests were conducted to evaluate whether the OEO retained its antioxidant capacity during incorporation in chitosan films and to determine their optimum concentration. Chitosan films showed radical scavenging activity, which may be mainly attributed to the capacity of residual free amino groups of chitosan to react with free radicals forming stable macromolecular radicals and ammonium groups (30).
Films with OEO exhibited a moderate level of radical scavenging activity. In the present work, the increased antioxidant activity occured because of the effectiveness of the OEO incorporated within chitosan films, and dependent on its concentration. Earlier studies in this laboratory (3) have shown that OEO is a potent antioxidant and its radical-scavenging activity (48-94%) was comparable to that of the standard synthetic (89%), TBHQ (tertiary butylhydroquinone). Therefore, the antioxidative effect of chitosan was enhanced by the addition of OEO.
Because the film with 2% of OEO presented the highest % of sequestration, it was decided to continue working with this concentration.
Agar diffusion method
Effects of OEO addition on antimicrobial properties of chitosan solution are shown in table 2 (page 180).
Table 2. Antibacterial activity (inhibitory zone) of OEO, chitosan solution, and their combination.
Tabla 2. Actividad antimicrobiana (zona de inhibición) del AEO, solución de quitosano y su combinación.

Pure edible coatings solutions and pure OEO were served as control to determine the potential antimicrobial effects of these solutions per se.
Solutions containing only chitosan were not effective against Gram-negative tested bacteria. These results agree with Fernandez-Saiz, et al. (2009), who reported that chitosan showed stronger bactericidal effects against Gram-positive bacteria than Gram-negative bacteria, at a concentration of 0.1% in agar medium.
Although available literature information varies, and occasionally, contradictory findings have been reported, it is generally recognized that yeasts and moulds are the most sensitive group to chitosan, followed by Gram-positive (1). Essential oils extracted from spices and herbs are generally recognized as containing active antimicrobial compounds. Carvacrol and thymol are phenolic compounds in oregano with antimicrobial activity (27).
The essential oils were more effective against gram-positive bacteria than the gram-negative bacteria. This difference is due to the cell wall structures of bacteria. Gram-negative cells should be more resistant because they possess lipopolysaccharides in the outer membrane, which protects them from hydrophobic components of the EOs. Gram-positive bacteria have a thicker peptidoglycan layer but lack the outer membrane and may be more permeable, interrupting the proton motive force, the electron flow, the active transport and the coagulation of cellular content (17). Among the bacteria examined, Listeria, Staphylococcus aureus, Bacillus cereus, Lactobacillus were the most susceptible to OEO. Incorporation of OEO into chitosan solutions at higher than 1% (v/v) exhibited a clear inhibitory area by the absence of bacterial growth with diameters larger than 8mm for Listeria, Salmonella, S. aureus, Bacillus and Lactobacillus. As the concentration increased, the area of inhibition did not increase. L. monocytogenes has the highest mortality rate (20-40%) in humans among other food-borne pathogens, affecting mainly those with underlying immune conditions, such as pregnant women, newborns, and elders, resulting in sepsis, meningitis, and/or meningoencephalitis. It is important to state that it can grow under refrigeration temperatures (4 to 10°C), which are commonly used to control pathogens in food (41).
On the other hand, the incorporation of 2% OEO to chitosanss solution did not reduce the growth of Gram negative bacteria like E. coli, Pseudomonas, Campylobacter, excep E. coli O157 when comparing with solutions without essential oil.
Lipid oxidation measurement
The effect of different treatments on TBARS value of beaf patties, over 9 days of refrigerated storage is shown in figure 1 (page 181).
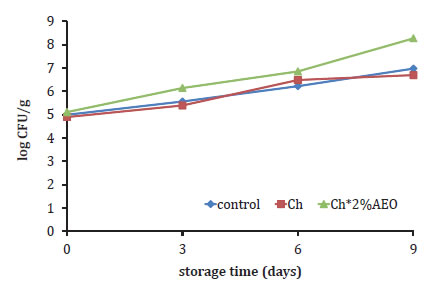
Figure 1. TBARS values of meat hamburger samples during storage at 4°C.
Figura 1. Valores de TBARS en las muestras de hamburguesas durante el almacenamiento a 4°C.
TBA values varied between 0.28 and 1.74 mg MDA kg-1 hamburger. These values are lower than the proposed limit of 2 mg MDA kg -1 above whose rancid off-flavours become sensorily detectible in meat products (8).
TBARS formation increased rapidly with storage time in control and samples coated with chitosan. In control, TBARS value was much higher than the values of the other treatments at the end of storage period (p < 0.05). Chitosan by itself has shown chelator antioxidant activity in cooked ground beef, and to inhibit warmed over flavor when mixed with the meat samples (42).
However, they were not effective, as shown in this study, when they were applied on the meat surface as coatings. These results were consistent with studies carried out by Wu, et al. (2000), who reported that chitosan coating in precooked beef patties were not effective in controlling lipid oxidation. Addition of the OEO showed significant (p < 0.05) effects compared to control and samples only with chitosan during storage of beef patties. This sample exhibited the lowest (p < 0.05) TBARS values. This may be due to strong antioxidant activity of spice extracts and synergistic effect with chitosan. Similar results were reported in fish (43).
pH
There was no significant difference between the samples treated (P<0.05) or storage time (data not shown). As all treatments have acetic acid, this solvent may be influencing the pH value, inhibiting the effect on the microorganisms that metabolize the nitrogenous basic compounds. An increase in pH would indicate an increase in volatile bases because microbial or endogenous enzymes use the amino acids present in the meat and decompose them to alkaline ammonium, e.g. trimethylamine and ammonia.
Color
Hunter color L*, a*, b* value of meat hamburger during storage is displayed in table 3.
Table 3. Color parameters (mean ± standard deviation) in meat hamburger during storage at 4°C.
Tabla 3. Valores de color (media ± desviación estándar) en hamburguesas durante su almacenamiento a 4°C.

The ANOVA for the L * data indicates that the values were significantly (P < 0.05) affected by the storage period.
In general, as regards control the storage of samples with chitosan increased lightness (L*), probably because chitosan coating increases light reflection on sample surface. During the first three or six days L* values of burgers increased, being the values higher in samples coating with only chitosan.
The above results, however, are in contrast to those of Chounou et al. (2013) which reported a decrease in lightness values in ground meat containing chitosan.
The increase in L*during the first days could be related to oxidation increasing metmyoglobin concentration (40). The subsequent decrease in L * may be due to oxidation of lipids that can increase permeability of the cell membrane and induce juice loss (37).
The major indicator of color stability in red meat and meat products is a* values.
The a* values of beef patties were significantly (p<0.05) affected by treatments and storage time. Samples containing chitosan in combination with OEO had higher a* values than others samples throughout the whole period of storage, indicating that chitosan coating together with OEO inclusion, delayed color deterioration.
Redness preservation due to the use of edible films with OEO has been linked to the control of oxidative changes due to the presence of EO. A decreasing trend was observed as regards to a* values, which is attributed to the gradual oxidation of myoglobin and accumulation of metmyoglobin with time (15).
For yellowness (b*), storage time and treatments had no significant (p<0.05) effect. The behavior of b* depends, to a great extent, on the food matrix, and it is known that changes (pH, oxidation extent, water activity, etc.) in the matrix have the greatest influence on this coordinate in many foods (14).
The discoloration of the patties was confirmed by calculation of the hue angle (H*). The use of H* is recommended for determination of meat discoloration and precise measure of color because human evaluators are better able to understand color (hue) and lightness (L*) (28).
Results demonstrated that control and Ch treatment had remarkably higher H* values (p<0.05) indicating these samples had lower a* and higher b* values than other formulation during the storage. The addition of 2% OEO significantly lowered the H* values and the effect became stronger with increasing storage time, which was attributed to antioxidant effect from OEO.
Microbiological analysis
No significant differences (p<0.05) were detected between samples or storage time in coliform bacteria amounts (data not shown). Coli fecal and E. coli did not develop in any of the samples tested.
YM amounts of the meat patties during storage are presented in figure 2 (page 184).

Figure 2. Growth of yeast and moulds in meat hamburger samples during storage at 4°C.
Figura 2. Crecimiento de levaduras y mohos en las muestras de hamburguesas durante su almacenamiento a 4°C.
In the present study, there was no significant difference among treatments. However, it showed a significant increasing trend during the entire storage period. Tsai, et al. (2002), described no antifungal activity in vitro for chitosan, at 2000 mg kg-1 against Aspergillus fumigatus or A. parasiticus.
Devlieghere,et al.(2004) explained that the effects of chitosan as an antimicrobial preservative for food will be limited to food products with low protein and NaCl content. Furthermore, Roller et al. (2002), reported that the addition of chitosan (0.6%) to sausages did not inhibit YM growth during storage at 4°C.
Aldemir and Bostan (2009), indicated that chitosan (50-500 mg kg-1) added into meatball was no effective on YM. However, in contrast to this findings Georgantelis et al. (2007), Petrou et al. (2012), reported sensitivity to chitosan for yeast and molds.
The PB for the different treatments are given in figure 3 (page 184), as a function of storage time.
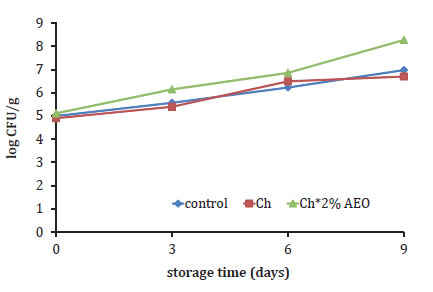
Figure 3. Growth of psychrotrophic bacteria in meat hamburger samples during storage at 4°C.
Figura 3. Crecimiento de bacterias psicrótrofas en las hamburguesas durante su almacenamiento a 4°C.
The initial PB value of control patty was 5 lg cfu g-1 and no significant differences were found with Ch treatments. All treatments showed slightly increasing bacteria numbers at the end of storage. Among all treatments, Ch+2% OEO had the higher PB during storage, and exceded the value of 7 log CFU g-1 for total bacterial counts on day 6 of storage, which was regarded as the upper acceptability limit for meat products (25), while Ch and control samples didn't reach the value during the whole 9 days storage. According to the results, the treatment with chitosan solution was not significantly different with the treatment control.
There are several explanations for the observed inactivity of chitosan against microorganisms on the meat surface.
The amount of chitosan might be insufficient for inhibition (27). Devlieghere et al. (2004), ilustrated that the effects of chitosan as an antimicrobial preservative for food will be limited to food products with low protein and NaCl content like these patties. Park et al.(2010), and Porter et al. (1995), indicated that high affinity for proteins and other substances may cause chitosan-meat complex formations on the meat surface.
These interactions between chitosan and meat components may reduce the amount of chitosan interacting with microorganisms on meat surface. Adding NaCl to the medium will also decrease the antimicrobial activity of chitosan because it interferes with the electrostatic forces between chitosan and the microbial surface.
On the one hand, the Cl- ions can neutralize the positive charges on the chitosan, while on the other hand, the Na+ ions can compete with chitosan for the negative charges on the cell surface (16). Some authors mention that these compounds are more effective in reducing microbial growth when the food is wrapped in the film than when applied to the surface by spraying or when directly added to the product because of the ability of the active substances of diffuse into the environment (18, 19).
Contrary to expectations, the addition of OEO to the coating significantly increased the count over the other two treatments. This can be attributed to the dilution effect of Ch when OEO is present, or to the bond of the active compounds in the Ch network through the strong interactions with the charged polymer chains, which made their access to the microorganisms difficult (39).
Results of this study (microbiological data) indicate that chitosan, applied via a dipping procedure, leads to a homogenous dispersion of this antimicrobial agent in the meat patties samples, without an efficient action against the microbiota, except coliforms and E. coli. Moreover, chitosan dipping when combined with OEO, does not result in antimicrobial properties when it is used as a coating for meat hamburgers.
Sensory characteristics
The results of the sensory evaluation (colour, odour, taste, and general acceptability) of meat patties are presented in table 4 (page 186).
Table 4. Results of sensory analyses of meat patties during storage at 4° C.
Tabla 4. Resultados del análisis sensorial de hamburguesas almacenadas a 4° C.
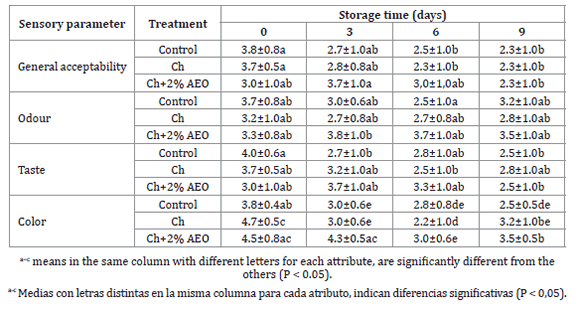
Gradual deterioration of all samples was observed. However, those containing Ch+2% OEO, deteriorated at a slower rate. Results of ANOVA showed that there were no significant differences among the three samples for general acceptability and showed that scores decreased with increasing storage periods. The effect of time can be appreciated from the third day of storage.
The sensory evaluation results appeared to correspond to microbial and chemical value analyses.
Samples coating with Ch+2% OEO could maintain acceptable odor quality until the end of the trial. Control samples reached the lower acceptability limit (score 3) on day 3-6 and samples with chitosan on day 0-3. On the initial day of storage, cooked patties had a pleasant taste.
The results, gave a sensorial shelf-life (based on taste acceptance scores) between days 0-3 for control, while Ch samples exceded the upper acceptability sensory limit (score 3) between days 3-6 of refrigerated storage. Moreover, Ch+2% OEO samples were sensorially acceptable between days 6-9.
Color evaluation showed significant changes due to formulation and storage time (p<0.05). Throughout refrigerated storage red/pink tones changed to brown tones in all samples. Burgers containing chitosan presented best visual appearance (red), especially those with 2% OEO. Present results, consistently agreed with instrumental color. Therefore, it is posible to say that Ch+OEO prolonged an acceptable odor, taste and color for more time than control and Ch.
CONCLUSIONS
The present work shows that origanum essential oil can be added to chitosan in a concentration of 2%, imparting a good antioxidant activity and increasing its antimicrobial efficacy in vitro. This combination showed the highest antimicrobial efficacy. Gram-positive bacteria were more susceptible than Gram-negative bacteria. These results may indicate its use as a natural preservative in foods against the causative agents of foodborne diseases and those of food spoilage.
In meat hamburguers, the results of this study indicate that chitosan coating with 2% of OEO had a significant antioxidant effect. However, no differences were observed between control and chitosan coating.
Considering conservation effects, differences in psychrotrophic bacteria values were not significant on the patties surfaces with chitosan or control and, surprisingly, their combination with OEO decreased the shelf life, being a health risk.
Significant extension of red color shelf life were observed in meat hamburguers coated with chitosan+2% OEO, and its application resulted in better odor and color characteristics of meat patties after long time storage, without affecting sensorial acceptability.
Nevertheless, further improvements are necessary to develop a more successful application of edible coatings enriched with OEO on meat hamburguers.
1. Aider, M. 2010. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT-Food Science and Technology. 43(6): 837-842. [ Links ]
2. Aldemir, T.; Bostan, K. 2009. Effects of chitosan on the microbiological quality of ready to cook meatball. İstanbul Üniversitesi Veteriner Fakültesi Dergisi. 35(2): 13-21. [ Links ]
3. Amadio, C. 2013. Genotipos de orégano de la provincia de Mendoza: caracterización físicoquímica y su empleo como aditivos alimentarios naturales (Tesis doctoral). Universidad Nacional de Cuyo. Mendoza. Argentina. [ Links ]
4. Amadio, C.; Medina, R.; Dediol, C.; Zimmermann, M. E., Miralles, S. 2011. Aceite esencial de orégano: un potencial aditivo alimentario. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 43(1): 237-245. [ Links ]
5. Amorati, R.; Foti, M.; Valgimigli, L. 2013. Antioxidant activity of essential oils. Journal of Agricultural and Food Chemistry. 61(46): 10835-10847. [ Links ]
6. Buege, J.; Aust, S. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52: 02-304. [ Links ]
7. Byun, Y.; Kim, Y.; Whiteside, S. 2010. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. Journal of Food Engineering, 100(2): 239-244.
8. Campo, M.; Nute, G.; Hughes, S.; Enser, M.; Wood, J.; Richardson, R. 2006. Flavour perception of oxidation in beef. Meat Science. 72(2): 303-311. [ Links ]
9. Caner, C.; Cansiz, O. 2007. Effectiveness of chitosan-based coating in improving shelf-life of eggs. Journal of the Science of Food and Agriculture. 87: 227-232. [ Links ]
10. Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. 2013. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food chemistry. 141(3): 1655-1660. [ Links ]
11. Chi, S.; Zivanovic, S.; Penfield, M. 2006. Application of chitosan films enriched with oregano essential oil on bologna–active compounds and sensory attributes. Food Science and Technology International. 12(2): 111-117.
12. Chouliara, E.; Karatapanis, A.; Savvaidis, I.; Kontominas, M. 2007. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4°C. Food Microbiology. 24(6): 607-617. [ Links ]
13. Chounou, N.; Chouliara, E.; Mexis, S.; Stavros, K.; Georgantelis, D.; Kontominas, M. 2013. Shelf life extension of ground meat stored at 4°C using chitosan and an oxygen absorber. International Journal of Food Science & Technology. 48(1): 89-95. [ Links ]
14. Cofrades, S.; Serrano, A.; Ayo, J.; Solas, M.; Carballo, J.; Jiménez-Colmenero, F. 2004. Restructured beef with different proportions of walnut as affected by meat particle size. Eur Food Res Technol. 218: 230-236. [ Links ]
15. Comi, G.; Tirloni, E.; Andyanto, D.; Manzano, M.; Iacumin, L. 2015. Use of bio-protective cultures to improve the shelf-life and the sensorial characteristics of commercial hamburgers. LWT-Food Science and Technology. 62(2): 1198-1202. [ Links ]
16. Devlieghere, F.; Vermeulen, A.; Debevere, J. 2004. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food microbiology. 21(6): 703-714. [ Links ]
17. dos Santos, N.; Aguiar, A. J.; de Oliveira, C.; de Sales, C.; Silva, S.; da Silva, R.; de Souza, E. 2012. Efficacy of the application of a coating composed of chitosan and Origanum vulgare L. essential oil to control Rhizopus stolonifer and Aspergillus niger in grapes (Vitis labrusca L.). Food microbiology. 32(2): 345-353. [ Links ]
18. Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. 2009. Perspectives for chitosan based antimicrobial films in food applications. Food chemistry. 114(4): 1173-1182. [ Links ]
19. Elsabee, M.; Abdou, E. 2013. Chitosan based edible films and coatings: a review. Materials Science and Engineering: C. 33(4): 1819-1841. [ Links ]
20. Fernandez-Saiz, P.; Lagaron, J.; Ocio, M. 2009. Optimization of the biocide properties of chitosan for its application in the design of active films of interest in the food area. Food Hydrocolloids. 23: 913-921. [ Links ]
21. Friedman, M.; Juneja, V. 2010. Review of antimicrobial and antioxidative activities of chitosans in food. Journal of Food Protection. 73(9): 1737-1761. [ Links ]
22. Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D. 2007. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Science. 75(2): 256-264.
23. Hawashin, M. D.; Al-Juhaimi, F.; Ahmed, I. A. M.; Ghafoor, K.; Babiker, E. E. 2016. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat science. 122: 32-39. [ Links ]
24. ICMSF. 2000. Microorganisms in Foods I. Their significance and methods of enumeration, 2° ed. University of Toronto Press. [ Links ]
25. Kanatt, S. R.; Rao, M. S.; Chawla, S. P.; Sharma, A. 2013. Effects of chitosan coating on shelflife of ready-to-cook meat products during chilled storage. LWT-Food science and technology. 53(1): 321-326. [ Links ]
26. Krkić, N.; Šojić, B.; Lazić, V.; Petrović, L.; Mandić, A.; Sedej, I.; Tomović, V. 2013. Lipid oxidative changes in chitosan-oregano coated traditional dry fermented sausage Petrovská klobása. Meat science. 93(3): 767-770.
27. Liu, N.; Chen, X.; Park, H.; Liu, C.; Liu, C.; Meng, X.; Yu, L. 2006. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydrate polymers. 64(1): 60-65. [ Links ]
28. Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F. 2015. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Science. 110: 93-100. [ Links ]
29. Martins, J.; Cerqueira, M.; Vicente, A. 2012. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocolloids. 27(1): 220-227.
30. Mokhtar, S.; Youssef, K.; Morsy, N. 2014. The effects of natural antioxidants on colour, lipid stability and sensory evaluation of fresh beef patties stored at 4°C. Journal of Agroalimentary Processes and Technologies. 20(3): 282-292. [ Links ]
31. Nychas, G. J. E.; Skandamis, P. N.; Tassou, C. C.; Koutsoumanis, K. P. 2008. Meat spoilage during distribution. Meat science. 78(1): 77-89. [ Links ]
32. Park, S.; Marsh, K.; Dawson, P. 2010. Application of chitosan-incorporated LDPE film to sliced fresh red meats for shelf life extension. Meat Science. 85(3): 493-499. [ Links ]
33. Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I. 2012. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. International journal of food microbiology. 156(3): 264-271. [ Links ]
34. Ponce, A.; Roura, S.; del Valle, C.; Moreira, M. 2008. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: in vitro and in vivo studies. Postharvest biology and Technology. 49(2): 294-300. [ Links ]
35. Porter, W.; Conca, K.; Lachica, R.; Mayer, J.; Pariser, E. 1995. Chitin and chitosan as novel protective food ingredients. Proceedings of 1995 meetings, activities report of the R&D associates. 47: 252-262. [ Links ]
36. Reda, W.; Abdel-Moein, K.; Hegazi, A.; Mohamed, Y.; Abdel-Razik, K. 2016. Listeria monocytogenes: An emerging food-borne pathogen and its public health implications. The Journal of Infection in Developing Countries. 10(02): 149-154. [ Links ]
37. Ripoll, G.; Joy, M.; Muñoz, F. 2011. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat science. 87(1): 88-93. [ Links ]
38. Roller, S.; Sagoo, S.; Board, R.; O'mahony, T.; Caplice, E.; Fitzgerald, G.; Fletcher, H. 2002. Novel combinations of chitosan, carnocin and sulphite for the preservation of chilled pork sausages. Meat Science. 62(2): 165-177. [ Links ]
39. Sánchez-González, L.; Cháfer, M.; Hernández, M.; Chiralt, A.; González-Martínez, C. 2011. Antimicrobial activity of polysaccharide films containing essential oils. Food Control, 22(8): 1302-1310. [ Links ]
40. Sayas-Barberá, E.; Quesada, J.; Sánchez-Zapata, E.; Viuda-Martos, M.; Fernández-López, F.; Pérez-Alvarez, J. A.; Sendra, E. 2011. Effect of the molecular weight and concentration of chitosan in pork model burgers. Meat science. 88(4): 740-749. [ Links ]
41. Tsai, G.; Su, W.; Chen, H.; Pan, C. 2002. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fisheries science. 68(1): 170-177. [ Links ]
42. Turgut, S. S.; Soyer, A.; Işıkçı, F. 2016. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat science. 116: 126-132. [ Links ]
43. Vatavali, K.; Karakosta, L.; Nathanailides, C.; Georgantelis, D.; Kontominas, M. 2013. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food and Bioprocess Technology. 6(12): 3510-3521. [ Links ]
44. Wu, Y.; Rhim, J.; Weller, C.; Hamouz, F.; Cuppett, S.; Schnepf, M. 2000. Moisture loss and lipid oxidation for precooked beef patties stored in edible coatings and films. Journal of food science. 65(2): 300-304. [ Links ]
ACKNOWLEDGMETS
This work was supported by Research and Technology Council (SECyT) of Cuyo University (UNCUYO), Mendoza, Argentina.














