Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
versión impresa ISSN 1853-8665versión On-line ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.51 no.2 Mendoza dic. 2019
ORIGINAL ARTICLE
Resistance to Meloidogyne enterolobii in sweet potatoes
Resistencia a Meloidogyne enterolobii en batatas
Ranoel José de Sousa Gonçalves 1, Regis de Castro Carvalho 2*, Wilson Roberto Maluf 2, Thiago Matos Andrade 3, Álvaro Carlos Gonçalves Neto 4, Maria Zilderlânia Alves 1
1 Academic Unit of Technology, Federal University of Campina Grande. Sumé. Paraíba. Brazil.
2 Federal University of Lavras. CEP. 37200-000. Lavras, Minas Gerais. Brazil. * regisccarvalho@hotmail.com
3 Federal University of Sergipe. São Cristóvão. Sergipe. Brazil.
4 Departament of Agricultural Science. Federal University of Paraíba. Bananeiras. Paraíba. Brazil.
Originales: Recepción: 01/03/2018 - Aceptación: 11/09/2018
ABSTRACT
The present work was designed to select for sweet potatoes clones (Ipomoea batatas) resistant to Meloidogyne enterolobii (Syn. M. mayaguensis) as well as evaluate the efficiency of the selection methods used by estimating their genetic (VCg) and environmental (VCe) variation coefficients as well as broad sense heritability. A total of 142 sweet potato genotypes were tested, including four commercial varieties (Brazlândia Rosada, Brazlândia Roxa, Brazlândia Branca, and Palmas) as well as the Santa Clara tomato cultivar (utilized as a susceptibility standard). The experimental design was completely randomized blocks, in two repetitions of six plants each. Resistance levels were classified according to the numbers of eggs per gram of roots, the reproduction factor (RF), and the reproduction index (RI) relative to the Santa Clara tomato cultivar. The b= VCg/VCe ratio and broad sense heritability were high in terms of the numbers of eggs per gram of roots, as well as in terms of the reproduction factor and reproduction index, demonstrating the efficiency of the methodology used in the selection of resistant genotypes. Thirty-one sweet potato genotypes resistant to M. enterolobii were identified as having significant potential for continuing the breeding program.
Keywords: Ipomoea batatas; Plant breeding; Reproduction index; Root-knot nematode
RESUMEN
Este trabajo fue realizado con el objetivo de seleccionar clones de batata (Ipomoea batatas) resistentes a Meloidogyne enterolobii (Syn. M. mayaguensis) y evaluar la eficiencia del método de selección empleado, por la estimación de los coeficientes de variación genética (CVg) y el medio ambiente (CVe), y de las estimaciones de las herencias en el sentido amplio. Se utilizaron 142 genotipos de batata, entre ellos cuatro cultivares comerciales: Brazlândia Rosada, Brazlândia Roxa, Brazlândia Branca y Palmas, y el tomate cv. Santa Clara (utilizado como patrón de susceptibilidad). El diseño experimental utilizado fue de bloques aleatorizados completos con dos repeticiones de seis plantas cada uno. La clasificación de los niveles de resistencia fue realizada de acuerdo con el número de huevos por gramo de raíz, factor de reproducción (FR) e índice de reproducción (IR) relativo a lo observado en el tomate Santa Clara. La relación b = CVg / CVe y la heredabilidad en sentido amplio fueron altas tanto para el número de huevos por gramo de raíz como para el factor de reproducción e índice de reproducción, demostrando la eficiencia del método empleado para la selección de genotipos resistentes. Se identificaron, como prometedores para dar continuidad al programa de mejoramiento genético, 31 genotipos de batata resistentes a M. enterolobii.
Palabras clave: Ipomoea batatas; Mejoramiento genético vegetal; Indice de reproducción; Nematodo del nudo
INTRODUCTION
Sweet potato [Ipomoea batatas (L.) Lam.] is one of the most valuable crops cultivated in tropical and subtropical regions for diverse forms of use (22). Sweet potato has multiple uses as food sources for humans (cooked sweet potatoes), or they can be processed industrially to produce starch and flower. They can also be used in animal feeds or as an alternative source of ethanol as a biofuel (1, 3, 7, 15).
Even though sweet potatoes have the potential for high production levels in Brazil, there productivity is generally low, principally due to the use of obsolete and degenerated genetic material that can make them susceptible to pests and diseases (1, 11). Root-knot nematodes (Meloidogyne spp.) are one of the major pathogens responsible for the low productivity of this staple in Brazil (13.5 t.ha-1) (6, 10).
The principal nematode species that infect sweet potato are M. javanica and M. incognita, races 1, 2, 3 and 4. Although sweet potatoes are considered "false non-hosts", as they frequently do not display symptoms most characteristic of those pathogens (galls, produced by females ovipositioning on the roots), the secondary roots of these plants can host dense populations of nematodes and the females will penetrate the roots (principally secondary roots) to deposit their egg masses (2, 6).
The use of nematicides as a control method is expensive and inefficient, and the inadequate application of these chemicals can contaminate workers, the sweet potatoes to be consumed, and the general environment (2).
As such, the use of resistant genotypes is indicated for controlling these soil pathogens as this technique does not increase production costs and does not impact the environment (5, 24).
A number of sweet potato varieties have demonstrated resistance to species of Meloidogyne knot-root nematodes, such as M. incognita (races 1, 2, 3 and 4) and M. javanica (12, 23), although new species of these pests have been described that are able to overcome plant defenses (12, 14, 17).
Among the new nematode species with potential for infecting and damaging sweet potatoes cultivars that demonstrate resistance to M. incognita and/or M. javanica, is Meloidogyne enterolobii (Syn. M. mayaguensis). The occurrence of M. enterolobii (26) was reported for the first time in Brazil in Petrolina (PE). In addition to its ability to disseminate quite rapidly, M. enterolobii is polyphagic and has been reported parasitizing ornamental plants, tobacco, soybeans, coffee trees, papaya plants, acerola, araçá, and a number of other horticultural plants (8, 25).
There is currently only scarce information available in Brazil concerning the resistance of commercial cultivars of sweet potatoes to M. enterolobii (4). Melo et al. (2011) evaluated the resistance of various genotypes of tomatoes, lettuce, common beans, peppers, green peppers, and sweet potatoes to M. enterolobii and reported that not a single tomato genotype analyzed (with or without the Mi allele that confers resistance to M. incognita or M. javanica) was resistant to that nematode. Only one cultivar of beans, two varieties of Capsicum chinense, and three of Capsicum annuum demonstrated moderate resistance levels.
Only five cultivars of lettuce and two clones of sweet potaoes (UFLA07-49 and UFLA07-53) demonstrated high resistance. Therefore, considering the aggressiveness of M. enterolobii and its capacity to infect a wide number of hosts, it will be extremely important to identify cultivars or additional varieties of sweet potatoes with resistance to this predator.
The present work therefore sought to identify sweet potato clones resistant to M. enterolobii and evaluate the efficiency of the selective methods used by estimating the genetic and environmental variation coefficients of those plants as well as heritability (in the broad sense).
MATERIAL AND METHODS
The present research was undertaken at the Horticulture Experimental Station of HortiAgro Sementes S.A., Fazenda Palmital, in the municipality of Ijaci, Minas Gerais State, Brazil (-21°14'16" x -45°08'00"; 918 m a. s. l.). Sweet potato clones with preliminary productivities evaluated at near or above 50 t of roots per hectare (116 total varieties) were identified and denominated 2007HSF-xxxyy, where xxx = number of the family of half-sibs and yy = the clone number selected within the family (table 2, page 324; table 3 page 325-326 and table 4, page 327-328).
Table 2. Average numbers of Meloidogyne enterolobii eggs per gram of roots (1) of 142 sweet potato genotypes and the Santa Clara tomato cultivar, utilized as the standard control for susceptibility.
Tabla 2. Promedio de huevos de Meloidogyne enterolobii por gramo de raíces (1) de 142 genotipos de batata y el cultivar de tomate Santa Clara, utilizado como control estándar para la susceptibilidad.
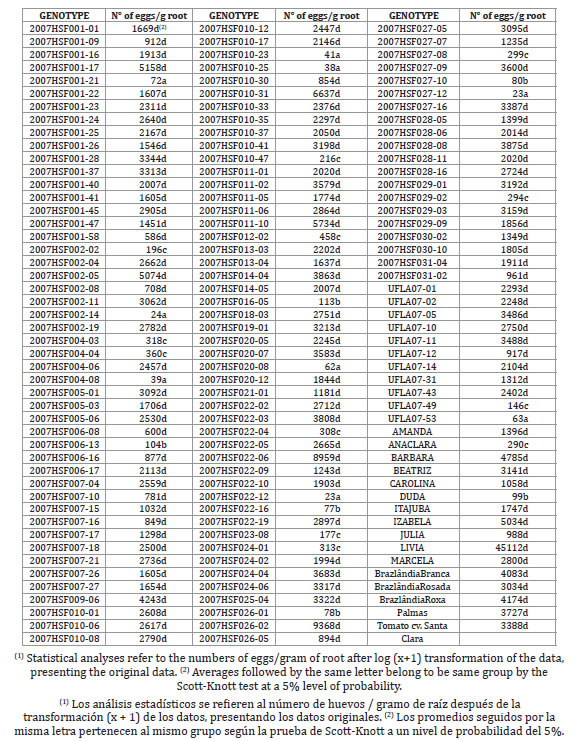
Table 3. Averages of the reproduction factor (RF) of Meloidogyne enterolobii in the 142 sweet potato genotypes evaluated and the Santa Clara tomato cultivar, and the classification of these genotypes in terms of their resistance (R) and susceptibility (S) to nematodes.
Tabla 3. Promedios del factor de reproducción (FR) de Meloidogyne enterolobii en los 142 genotipos de batata evaluados y el cultivar de tomate Santa Clara, y la clasificación de estos genotipos en términos de su resistencia (R) y susceptibilidad (S) a los nematodos.
Table 3 (cont.). Averages of the reproduction factor (RF) of Meloidogyne enterolobii in the 142 sweet potato genotypes evaluated and the Santa Clara tomato cultivar, and the classification of these genotypes in terms of their resistance (R) and susceptibility (S) to nematodes.
Tabla 3 (cont.). Promedios del factor de reproducción (FR) de Meloidogyne enterolobii en los 142 genotipos de batata evaluados y el cultivar de tomate Santa Clara, y la clasificación de estos genotipos en términos de su resistencia (R) y susceptibilidad (S) a los nematodos
Table 4. Averages of the reproduction indices (RI) of Meloidogyne enterolobii and their classification in terms of resistance to 142 sweet potato genotypes and the Santa Clara tomato cultivar (utilized as the standard control for susceptibility).
Tabla 4. Promedios de los índices de reproducción (IR) de Meloidogyne enterolobii y su clasificación en términos de resistencia a 142 genotipos de batata y el cultivar de tomate Santa Clara (utilizado como control estándar para la susceptibilidad).
Table 4 (cont.). Averages of the reproduction indices (RI) of Meloidogyne enterolobii and their classification in terms of resistance to 142 sweet potato genotypes and the Santa Clara tomato cultivar (utilized as the standard control for susceptibility).
Tabla 4 (cont.). Promedios de los índices de reproducción (IR) de Meloidogyne enterolobii y su clasificación en términos de resistencia a 142 genotipos de batata y el cultivar de tomate Santa Clara (utilizado como control estándar para la susceptibilidad)
A total of 142 sweet potato genotypes were examined (table 2, page 324; table 3 page 325-326 and table 4, page 327-328), including: four commercial cultivars (Brazlândia Rosada, Brazlândia Roxa, Brazlândia Branca, and Palmas), the 116 clones of sweet potatoes 2007HSF-xxx-yy, 11 varieties (denominated UFLA-07-01, UFLA-07-02, UFLA-07-05, UFLA-07-10, UFLA-07-11, UFLA-07-12, UFLA-07-14, UFLA-07-31, UFLA-07-43, UFLA-07-49, and UFLA-07-53, and 11 clones (denominated Amanda, Anaclara, Barbara, Beatriz, Carolina, Duda, Itajubá, Izabela, Julia, Livia, and Marcela) from among cultivars being examined by the breeding program at the Tocantins Federal University. The Santa Clara tomato cultivar was used as the standard nematode host.
Styrofoam trays (with 72 wells) containing a commercial substrate (approximately 120 ml of substrate per cell) were used to plant branches approximately 20 cm long with 3 to 4 internodal buds. Nematode inoculation was performed 30 days after sowing, using M. enterolobii eggs extracted from previously inoculated tomato plants (cv. TOM-684, containing the Mi gene and susceptible to M. enterolobii), following Hussey and Barker (1973). After washing, the tomato plant roots were cut into small pieces, processed in a blender for 30 seconds in a solution of 0.5% sodium hypochlorite, and subsequently passed through a 0.074 mm sieve (200 mesh) and retained on a smaller sieve (0.028 mm / 500 mesh) in abundant water. The 0.074 mm sieve retained the remnants of the tomato roots while the 0.028 mm sieve collected the eggs of M. enterolobii, which were subsequently transferred to a holding beaker (using pure water).
The water level in the beaker was then completed to 1000 ml, and three 1 ml volumes were removed from the homogenized slurry of eggs with the aid of a pipette and subsequently counted in a Peters chamber using a stereomicroscope. An aliquot of this suspension containing 2000 eggs was then used to inoculate the sweet potato plants (using a veterinary syringe to inject eggs in the commercial substrate).
The viability of the inoculum was evaluated in an eclosion chamber, indicating that 63.95% of the eggs were viable, corresponding to an initial population of 1,279 viable eggs per inoculum.
The plants were treated in two repetitions of six plants each. The plants were arranged in trays with 11 rows holding six plants each, with rows of the Santa Clara tomato cultivar alternating with different sweet potato genotypes. As such, one repetition represented a trial with six plants. Sixty days after inoculation with the nematode eggs, the plants were carefully removed from the polystyrene trays and their roots washed to extract the eggs. The numbers of eggs were estimated by counting 1 mL of the suspension in a Peters chamber, using a stereomicroscope. The total numbers of collected eggs were determined by extrapolating from these sample counts.
The parameters used as resistance parameters were: numbers of eggs per gram of roots; reproduction factor (RF = final population / initial population of viable eggs); and the reproduction index. To calculate the number of eggs per gram of roots, the total number of eggs extracted from each radicular system was divided by the fresh mass of the roots. The reproduction factor (RF) was used to define resistance (RF<1) and susceptibility (RF≥1), following Oostenbrink (1966).
The reproduction index of M. enterolobii was determined using tomato plants as standard controls (100%). The values of the final populations (Pf) encountered in the sweet potato genotypes were divided by the populations encountered in the tomato controls. Based on these values, it was could defined the levels of resistance of each sweet potato genotype to M. enterolobii according to the reproduction index (RI) established by Taylor (1967), in which: S = a susceptible plant (normal reproduction), ratio > 51% in relation to the tomato standard controls; SR = slightly resistant, from 26% to 50%; MoR = moderately resistant, from 11% to 25%; VR = very resistant, from 1% to 10%; HR/I = highly resistant/immune, <1%.
To attend the prerequisites of analyses of variance, the values obtained for all of the parameters were submitted to log transformation (x+1), in which "x" resents the number of eggs per gram of roots. The transformed data was then submitted to analysis of variance using the SAS software package (18). The averages of the treatments were compared using the Scott and Knott test. Based on the expected mean squares of the analysis of variance, it was estimated the genetic (σ g 2) and environmental (σe2) variances and broad sense heritability (ha2) for each character, as described by Tsegaye et al. (2007), using the following equation:
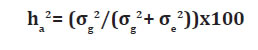
The genetic (VCg) and environmental (VCe) variation coefficients, as well as the b index (VCg/VCe) for the characters evaluated were estimated (21), based on the following expressions:
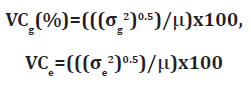
RESULTS AND DISCUSSION
There were significant differences between the numbers of eggs per gram of roots, the reproduction factors (RF), and the reproduction indices (RI) of M. enterolobii among the sweet potato genotypes, indicating significant genetic variability among those plants (table 1, page 323).
Table 1. Results of the analyses of variance of the numbers of eggs per gram of root, reproduction factor (RF), and reproduction index (RI) of Meloidogyne enterolobii on the roots of sweet potato clones.
Tabla 1. Resultados de los análisis de varianza de los números de huevos por gramo de raíz, factor de reproducción (FR) e índice de reproducción (IR) de Meloidogyne enterolobii en las raíces de los clones de batata.

In this respect, it is important to emphasize that the contribution of epigenetic mechanisms involved cannot be ruled out. These may be defined as those mechanisms that cause changes in the genome, although inheritable during cell division, but which do not involve a change in the DNA sequence.
According to Tang and Ho (2007), epigenetic patterns are sensitive to environmental changes that may cause phenotypic changes that will be transmitted to offspring, which do not alter the DNA sequence but are inheritable by mitosis and over generations.
The estimates of the coefficients of environmental variation (VCe) were 7.69%, 22.03% and 11.05%, for the number of eggs per gram of roots, the reproduction factor (RF), and the reproduction index (RI) respectively. These estimates of the coefficient of variation were inferior to those commonly observed in experiments examining nematode resistance in sweet potatoes (13). Very elevated estimates for the coefficients of genetic variation (VCg) were observed in the present work as compared to the estimates of VCe, these being 18.90%, 48.21% and 32.20% for the numbers of eggs per gram of roots, the reproduction factor, and the reproduction index respectively. These results imply that there is a high degree of variability among the samples, and therefore very favorable conditions for the selection of resistant clones - as seen by the values of the ratio b= VCg/VCe, which were greater than 2.0 (table 1, page 323) for the three characteristics analyzed.
The estimates of broad sense heritability (ha2) were high (above 80%) for the three variables analyzed (table 1, page 323), reinforcing the probability that most of the phenotypic variability was of a genetic nature, and indicating that selection based on these characteristics could be undertaken with efficiency, as was noted above. Similar values of heritability were also described by Andrade Júnior et al. (2016), who evaluated sixty-three clones of sweet potato for resistance to Meloidogyne javanica. Considering the both classification criteria (reproduction factors and reproduction index), 71.43% of sweet potato clones were classified as resistant.
Based on the criterion of the numbers of eggs per gram of roots, 80.99% of the genotypes tested were considered susceptible, as their parameters did not differ significantly by the Scott-Knott test at a 5% level of probability from those of the Santa Clara tomato cultivar used as a susceptible control (table 2, page 324).
The sweet potato cultivars 'Brazlândia Rosada', 'Brazlândia Roxa', 'Brazlândia Branca', and 'Palmas' tested here were found to be susceptible to M. enterolobii. Marchese et al. (2010) noted that the cultivars Palmas and Brazlândia Roxa were resistant to race 1 of M. incognita, while 'Brazlândia Rosada' and 'Brazlândia Branca' were susceptible to it. Resistance to M. incognita was therefore not found to be associated with resistance to M. enterolobii. Twenty-seven sweet potato genotypes (19.01%) were classified as resistant to M. enterolobii as they fell into groups that were distinct from the Santa Clara tomato cultivar, by the Scott-Knott test at a 5% level of probability (table 2, page 324).
Fully 78.87% of the sweet potato genotypes tested were considered susceptible by the criteria used by Oostenbrink (1966), with their reproduction factors being equal to or more than 1.0 (table 3, page 325-326); these results were likewise confirmed by the numbers of eggs per gram of roots for the cultivars Brazlândia Branca, Brazlândia Roxa, Brazlândia Rosada, and Palmas.
It was encountered susceptible to highly resistant or immune genotypes according to their reproduction indices and the reproduction criteria established by Taylor (1967), (table 4, page 327-328). Among the genotypes tested, 78.16% (S= susceptible and LR= slightly resistant) were classified as susceptible, including the varieties Brazlândia Branca, Brazlândia Roxa, Brazlândia Rosada, and Palmas; 4.93% were classified as moderately resistant; 15.49% as very resistant; and 1.40% as highly resistant or immune (table 4, page 327-328).
The clones UFLA-07-49 and UFLA-07-53 were among the genotypes most resistant to the nematode M. enterolobii according to the three criteria utilized. The clones denominated UFLA-07-xx represent genotypes that have already been tested in advance phases of the breeding program at UFLA and demonstrated high productivity and commercially interesting attributeswhich, summed with their resistance to the nematode M. enterolobii, make them very promising for commercial markets. In recent studies, these same two genotypes demonstrated resistance to the nematode M. incognita race 1 (13).
Using the criteria established by Taylor (1967), 31 genotypes could be selected for further study as they represent clones that are highly resistant/immune, very resistant, and moderately resistant to M. enterolobii (table 4, page 327-328). Marchese et al. (2010), in their search for sweet potato genotypes resistant to M. incognita race 1, considered only plants that were highly resistant/immune or very resistant as worthy of selection. However, that study did not use multiple comparison procedures that might recommend the inclusion of moderately resistant genotypes.
In comparing the reproduction factor with reproduction index criteria, it was observed that genotype 2007HSF001-58 would not be considered resistant according to the first criterion, even though demonstrated an average RF of 1.09 (table 3, page 325-326), a value very close to the cutoff value for selected genotypes; it was, however, considered moderately resistant according to the criteria established by Taylor (1967).
Twenty-seven genotypes (19.01%) were classified as resistant according to all three selection criteria considered together (tables 2, page 324; table 3, page 325-326 and table 4, page 327-328).
The genotypes 2007HSF001-58, 2007HSF002-08, 2007HSF006-08, and the Carolina variety did not show consistency in terms of their resistance according to all of the criteria used, and would not be selected based on the numbers of eggs per gram of roots (table 2, page 324).
However, comparisons of the averages of these genotypes with that of the Lívia genotype indicated considerable differences between them, as the populations of nematodes in the latter grew by more than 30X, as was noted above.
In general, the three criteria were coherent among themselves and efficient in the identification and selection of genotypes resistant to M. enterolobii. It could be observed, however, that the reproduction index generated a wider distribution of distinct classes (AR/I, MR, MoR, LR and S), allowing more flexibility in establishing a cut-off level for genotype selection. As such, we selected 31 genotypes with some level of resistance according to the reproduction index criterion to continue the sweet potato breeding program.
Of the 142 sweet potato genotypes evaluated in the present study, 121 had been used in experiments undertaken by Marchese et al. (2010). Comparing these two studies indicated that the genotypes that demonstrated a certain degree of resistance to both race 1 of M. incognita and M. enterolobii were clones 2007HSF001-21, 2007HSF002-02, 2007HSF002-08, 2007HSF002-14, 2007HSF004-08, 2007HSF006-13, 2007HSF010-25, 2007HSF012-02, 2007HSF020-08, 2007HSF023-08, 2007HSF024-01, 2007HSF027-08, UFLA07-49, and UFLA07-53. These genotypes appear to be promising in terms of the sweet potato breeding program at UFLA, as the definition of genotypes that demonstrate resistance to large numbers of knot-root nematode species should generate more confidence among farmers and therefore more acceptance.
The observations that there are genotypes resistant to both M. incognita and M. enterolobii, and that some genotypes demonstrate resistance to race 1 of M. incognita but susceptibility to M. enterolobii, and vice-versa, indicate that the genes that confer resistance to M. incognita are not the same as those conferring resistance to M. enterolobii.
CONCLUSIONS
The relationships between estimates of the coefficients of genetic and environmental variation, and broad sense heritability were high in terms of the numbers of eggs per gram of roots as well as for the reproduction factors and reproduction indices, demonstrating the efficiency of the methodology used here for selecting resistant genotypes.
Thirty-one sweet potato genotypes resistant to M. enterolobii (21.84% of the clones evaluated) were selected for continuing the breeding program.
1. Andrade Júnior, V. C.; Viana, D. J. S.; Pinto, N. A. V. D.; Ribeiro, K. G.; Pereira, R. C.; Neiva, I. P.; Azevedo, A. M.; Andrade, P. C. R. 2012. Características produtivas e qualitativas de ramas e raízes de batata-doce. Horticultura Brasileira. Brasília. Brazil. 30(4): 584-589. Available in: http://dx.doi.org/10.1590/S0102-05362012000400004 [ Links ]
2. Andrade Júnior, V. C.; Gomes, J. A. A.; Oliveira, C. M.; Azevedo, A. M.; Fernandes, J. S. C.; Gomes, L. A. A.; Maluf, W. R. 2016. Resistência de clones de batata-doce a Meloidogyne javanica. Horticultura Brasileira. Brasília. Brazil. 34(1): 130-136. Available in: http://dx.doi. org/10.1590/S0102-053620160000100020 [ Links ]
3. Azevedo, A. M.; Andrade Júnior, V. C.; Viana, D. J. S.; Elsayed, A. Y.; Pedrosa, C. E.; Neiva, I. P.; Figueiredo, J. A. 2014. Influence of harvest time and cultivation sites on the productivity and quality of sweet potato. Horticultura Brasileira. Brasília. Brazil. 32(1): 21-27. Available in: http://dx.doi.org/10.1590/S0102-05362014000100004 [ Links ]
4. Cantu, R. R.; Wilcken, S. R. S.; Rosa, J. M. O.; Goto, R. 2009. Reação de porta enxertos comerciais de tomateiro a Meloidogyne mayaguensis. Summa Phytopathologica. Botucatu. Brazil. 35(3): 216-218. Available in: http://dx.doi.org/10.1590/S0100-54052009000300009 [ Links ]
5. Chaves, P. P. N.; Santos, G. R.; Silveira, M. A.; Gomes, L. A. A.; Momenté, V. G.; Nascimento, I. R. 2013. Reação de genótipos de batata-doce a nematóides de galhas em condições de temperatura elevada. Bioscience Journal. Uberlândia. Brazil. 29(6): 1869-1877. [ Links ]
6. Gomes, J. A. A.; Andrade Júnior, V. C.; Oliveira, C. M.; Azevedo, A. M.; Maluf, W. R.; Gomes, L. A. A. 2015. Resistance of sweet potato clones to Meloidogyne incognita races 1 and 3. Bragantia. Campinas. Brazil. 74(3): 291-297. Available in: http://dx.doi.org/10.1590/1678-4499.0454 [ Links ]
7. Gonçalves Neto, A. C.; Maluf, W. R.; Gomes, L. A. A.; Gonçalves, R. J. S.; Silva, V. F.; Lasmar, A. 2011. Aptidões de genótipos de batata-doce para consumo humano, produção de etanol e alimentação animal. Pesquisa Agropecuária Brasileira. Brasília. Brazil. 46(11): 1513-1520. Available in: http://dx.doi.org/10.1590/S0100-204X2011001100013 [ Links ]
8. Guimarães, L. M. P.; Moura, R. M.; Pedrosa, E. M. R. 2003. Parasitismo de Meloidogyne mayaguensis em diferentes espécies botânicas. Nematologia Brasileira. Brasília. Brazil. 27(2): 139-147. [ Links ]
9. Hussey, R. S.; Barker, K. R. 1973. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter. Saint Paul. USA. 57: 1025-1028. [ Links ]
10. IBGE - Instituto Brasileiro de Geografia e Estatística. 2015. Available in http:// biblioteca.ibge. gov.br/visualizacao/periodicos/66/pam_2015_v42_br.pdf. Accessed in April 10th, 2017. [ Links ]
11. Kalkmann, D. C.; Peixoto, J. R.; Nobrega, D. S. 2013. Reação de clones de batata-doce à Meloidogyne incognita raças 1 e 4 e estimativa de parâmetros genéticos. Horticultura Brasileira. Brasília. Brazil. 31(2): 293-296. Available in: http://dx.doi.org/10.1590/ S0102-05362013000200019 [ Links ]
12. López-Lima, D.; Carrión, G.; Sánchez-Nava, P.; Desgarennes, D.; Villain, L. (en prensa) Fungal diversity and Fusarium oxysporum pathogenicity associated with coffee corky-root disease in Mexico. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. [ Links ]
13. Marchese, A.; Maluf, W. R.; Gonçalves Neto, A. C.; Gonçalves, R. J. S.; Gomes, L. A. A. 2010. Seleção de clones de batata-doce resistentes a Meloidogyne incognita raça1. Pesquisa Agropecuária Brasileira. Brasília. Brazil. 45(9): 997-1004. Available in: http://dx.doi. org/10.1590/S0100-204X2010000900009 [ Links ]
14. Melo, O. D.; Maluf, W. R.; Gonçalves, R. J. S.; Gonçalves Neto, A. C.; Gomes, L. A. A.; Carvalho, R. C. 2011. Triagem de genótipos de hortaliças para resistência a Meloidogyne enterolobii. Pesquisa Agropecuária Brasileira. Brasília. Brazil. 46(8): 829-835. Available in: http:// dx.doi.org/10.1590/S0100-204X2011000800007 [ Links ]
15. Oliveira, A. M. S.; Blank, A. F.; Alves, R. P.; Arrigoni-Blank, M. F.; Maluf, W. R.; Fernandes, R. P. M. 2017. Performance of sweet potato clones for bioethanol production in different cultivation periods. Horticultura Brasileira. Brasília. Brazil. 35(1): 57-62. Available in: http://dx.doi.org/10.1590/S0102-053620170109 [ Links ]
16. Oostenbrink, M. 1966. Major characteristics of the relation between nematodes and plants. Wageningen: Mededelingen Landbouwhogeschool Wageningen. Nederland. [ Links ]
17. Salas, A.; Rusconi, J. M.; Camino, N.; Eliceche, D.; Achinelly, M. F. 2017. First record of Diploscapter coronata (Rhabditida), a possible health significance nematode associated with tomato crops in Argentina. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 49(1): 167-173. [ Links ]
18. SAS Institute. 2001. SAS/STAT software: changes and enhancements. Release 8. Cary: SAS Institute. [ Links ]
19. Tang, W. Y.; Ho, S. M. 2007. Epigenetic reprogramming and imprinting in origins of disease. Rev. Endcr. Metab. Disord. Louisville. USA. 8(2): 173-182. Available in: https://doi. org/10.1007/s11154-007-9042-4 [ Links ]
20. Taylor, A. L. 1967. Introduction to research on plant nematology: an FAO guide to study and control of the plant-parasitic nematodes. Rome: Food And Agricultural Organization of the United Nations. [ Links ]
21. Tsegaye, E.; Dechassa, N.; Sastry, E. V. D. 2007. Genetic variability for yield and other agronomic traits in sweet potato. Journal of Agronomy. Faisalabad. Pakistan. 6(1): 94-97. http:// dx.doi.org/10.3923/ja.2007.94.99 [ Links ]
22. Viana, D. J. S.; Andrade Júnior, V. C.; Ribeiro, K. G.; Pinto, N. A. V. D.; Neiva, I. P.; Figueiredo J. A.; Lemos, V. T.; Pedrosa, C. E.; Azevedo, A. M. 2011. Potencial de silagens de ramas de batata-doce para alimentação animal. Ciência Rural. Santa Maria. Brazil. 41(8): 1466- 1471. Available in: http://dx.doi.org/10.1590/S0103-84782011000800027 [ Links ]
23. Wanderley, M. J. A.; Santos, J. M. 2004. Resistência de cultivares de batata-doce a Meloidogyne incognita. Fitopatologia Brasileira. Brasília. Brazil. 29(4): 437-440. Available in: http:// dx.doi.org/10.1590/S0100-41582004000400014 [ Links ]
24. Wesemael, W. M. L.; Viaene, N.; Moens, M. 2011. Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology. United Kingdom. 13(1): 3-16. Available in: http://dx.doi. org/10.1163/138855410X526831 [ Links ]
25. Westerich, J. N.; Rosa, J. M. O. R.; Wilcken, S. R. S. 2011. Estudo Comparativo da Biologia de Meloidogyne enterolobii (= M. mayaguensis) e Meloidogyne javanica em Tomateiros com Gene Mi. Summa Phytopathologica. Botucatu. Brazil. 37(1): 35-41. Available in: http://dx.doi.org/10.1590/S0100-54052011000100006 [ Links ]
26. Yang, B. J.; Eisenback, J. D. 1983. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. Journal of Nematology. Florida. USA. 15(3): 381-391. [ Links ]
ACKNOWLEDGEMENTS
Our thanks to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for granting scholarships; to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); to the Universidade Federal de Lavras - Fundação de Apoio ao Ensino, Pesquisa e Extensão, and Fundação de Desenvolvimento Científico e Cultural for financial resources and infrastructure; and to the company HortiAgro Sementes S.A. for support in carrying out the experiments.














