Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
Print version ISSN 1853-8665On-line version ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.52 no.2 Mendoza Dec. 2020
ORIGINAL ARTICLE
Yacon (Smallanthus sonchifolius), propagation from rhizophores with different numbers of buds
Propagación de yacon (Smallanthus sonchifolius), a partir de rizóforos con diferentes números de yemas
Joab Luhan Ferreira Pedrosa 1*, Fábio Luiz de Oliveira1, Moises Zucoloto1, Ariany das Graças Teixeira1, Magno do Carmo Parajara1, Marcelo Antonio Tomaz1
1 Universidade Federal do Espírito Santo. Departamento de Agronomia. Alto Universitário. s/nº Guararema. 29500-000 Alegre. Espírito Santo. Brazil. * joabhuhan@yahoo.com.br
Originales: Recepción: 06/03/2019 - Aceptación: 25/08/2020
ABSTRACT
Yacon (Smallanthus sonchifolius) is a plant of Andean origin currently cultivated and consumed in several parts of the world for having numerous nutraceutical properties. An increasing interest in its cultivation demands advanced technical information on this crop, still scarce. Considering this, our study aimed to evaluate the propagation of yacon by rhizophores with different bud number. The experiment was conducted in two phases: the first phase was developed in a seedling nursery, using a completely randomized design with four treatments and 50 repetitions. The treatments consisted of rhizophores with: one to two buds (T1), three to four buds (T2), five to six buds (T3), and seven to eight buds (T4). The second phase was performed in the field with random blocks, four treatments, four repetitions, and the same treatments of the first phase. The following morphological and growth characteristics were assessed: plant height, stem diameter, number of leaves, number of stems per plant, Dickson quality index (DQI), leaf area, leaf dry mass, rhizophores, tuberous root, and productivity. The following physiological features were also analyzed: chlorophyll content (FCI – Falker chlorophyll index), net carbon assimilation rate, leaf transpiration, stomatal conductance, internal carbon concentration, water use efficiency and instantaneous carboxylation efficiency. Seedlings from rhizophores with three to four buds presented the best morphological and growth characteristics, DQI = 0.47 and good performance in the field. In addition, the plants originated from this material (rhizophores with three to four buds) showed greater accumulation of dry matter in the aerial part and tuberous roots, and consequently higher productivity in fresh tuberous roots, constituiting a suitable propagation form for the cultivation of yacon.
Keywords: Asexual; Polymnia sonchifolia; Propagule; Production; Smallanthus sonchifolius
RESUMEN
Yacon (Smallanthus sonchifolius) es una planta de origen andino que actualmente se cultiva y consume en varias partes del mundo por tener numerosas propiedades nutracéuticas. El aumento en el interés por la especie ha generado una demanda de información técnica sobre su cultivo, sin embargo, esta información es escasa. Este artículo tiene como objetivo evaluar la propagación de yacon a partir de rizóforos con diferentes números de yemas. Así, el experimento se desarrolló en dos fases. El primero fue desarrollado en un vivero de plántulas, en un diseño completamente al azar, con cuatro tratamientos y 50 repeticiones. Los tratamientos consistieron en propágulos con: T1 (una a dos yemas), T2 (tres a cuatro yemas), T3 (cinco a seis yemas) y T4 (siete a ocho yemas). La segunda fase se realizó en campo con bloques aleatorios, con cuatro tratamientos y 4 repeticiones, teniendo los mismos tratamientos que la primera fase. Se evaluaron las siguientes características morfológicas y de crecimiento: altura de la planta, diámetro del tallo, número de hojas, cantidad de tallos por planta, índice de calidad de Dickson (DQI), área foliar, biomasa foliar, rizóforos, raíz tuberosa y productividad en raíces tuberosas frescas. Las características fisiológicas evaluadas fueron el contenido relativo de clorofila (FCI – índice de clorofila Falker), la tasa neta de asimilación de carbono, la transpiración de las hojas, la conductancia estomática, la concentración interna de carbono, la eficiencia del uso del agua y la eficiencia de la carboxilación instantánea. Las plántulas de rizóforo con tres a cuatro yemas presentaron el mejor rendimiento de características morfológicas y de crecimiento, un DQI = 0,47 y un buen rendimiento en el campo. Además, las plantas originarias de este material (rizóforos con tres y cuatro yemas) mostraron una mayor acumulación de materia seca en la parte aérea y en las raíces tuberosas, en consecuencia, mayor productividad en las raíces tuberosas frescas, mostrándose como una forma adecuada de propagación para el cultivo de yacon.
Palabras clave: Asexual; Polymnia sonchifolia; Propágulo; Producción; Smallanthus sonchifolius
INTRODUCTION
Yacon (Smallanthus sonchifolius) is a perennial herbaceous plant from the Asteraceae family, native of the Andean region of South America, that produces tuberous roots. Its roots have reduced amounts of starch and high concentrations of fructooligosaccharides (FOS) and inulin, bioactive compounds potentially beneficial to human health, especially for diabetics (3).
Although many biochemical and genetic aspects of this plant are well known (4, 16, 17, 32), the growing interest for yacon demands further physiological and agronomic information for its cultivation, especially related to its form of propagation.
Gas exchange makes it possible to detect which photosynthetic strategies are developed under different conditions. The net photosynthetic rate (A), for example, corresponds to the net carbon gain obtained by the difference between a plant’s photosynthetic and respiratory processes (40, 41). Thus, understanding the physiological characteristics of yacon can clarify the effects of the strategies used by this species for its growth and vegetative development. However, the physiological performance of a plant species is strongly affected by morphological variations (21, 23, 38). The propagation of yacon for agronomic purposes is basically vegetative, or asexual, since the plant has low sexual propagation efficiency, probably due to many causes, such as pollen infertility, environmental conditions during germination and the hybrid allopolyploid origin (15).
There are two ways of vegetative propagation for yacon: through herbaceous stem cuttings or through rhizophores. Propagation via stem cuttings consists of using vegetative stems of unsprouted plants. This is a less common technique, since, despite enabling the obtention of propagules in half of the time needed for rhizophores, rooting (number of roots) of plants generated by this technique could be lower than the one obtained by rhizophore propagation. Moreover, dehydration affecting the cuttings, reduces seedling sprouting, leading to non-uniformity in the field (31).
Traditionally, Yacon cultivation has been performed by planting rhizophores weighing between 60 and 80 g, which demands from 1800 to 2400 kg of rhizophores for each planted hectare, for a density of 30,000 plants per hectare (42). This situation increases implantation costs and constitutes a limiting factor for the expansion of this crop, concentrated in a specific season of the year.
Another point to be considered is that propagation should not be based on the average weight of the rhizophores as for other plants (25, 27, 39, 43). According to Hartmann et al. (2011) and Taiz et al. (2017), the number of buds is related to the ability for emitting shoots and to the future photosynthetic capacity, in addition to the production of substances such as auxins, an important rooting promoting factor. This has also been observed for other cultures, such as Colocasia esculenta (25), Arracacia xanthorrhiza (10), Xanthosoma sagittifolium (30) and Turnera subulata (5).
In this context, this study states the hypothesis that rhizophores used in the propagation of yacon can be selected by number of buds, and not by a single specific weight, and that the number of buds in the rhizophores can interfere with the establishment of seedlings and with their growth and vegetative development in the field. Therefore, to define the best propagule strategy based on the number of buds in the rhizophores, is important This research aimed to evaluate the propagation of yacon from rhizophores with different bud number.
MATERIALS AND METHODS
Development of seedlings in the nursery
The experiment was installed in a commercial nursery for seedling production, from February to April 2017, located in the municipality of Alegre, in the State of Espírito Santo, Brazil, (20°47’1” S, 41°36’56” W, 640 m a. s. l.). A completely randomized design, with four treatments and 50 repetitions (the first stem developed from each repetition was considered the experimental unit) was followed. Each treatment consisted of seedlings produced from rhizophores with different bud number: one to two buds (T1); three to four (T2); five to six (T3); and seven to eight (T4). The rhizophores weighted on average 20 g (T1), 30 g (T2), 40 g (T3), and 100 g (T4).
The rhizophores were obtained from an experimental yacon crop grown in the Alto Norte municipality of Muniz Freire/ES (1180 m a.s.l.). Afterward, the plant material was taken to the plant analysis laboratory at the Federal University of Espírito Santo (CCAE-UFES) in order to prepare the propagules. Before planting, the rhizophores were cut with gardening scissors and standardized by the number of buds, according to each treatment. To protect the propagules from pathogenic agents, the rhizophores were washed with running water and then immersed for 10 minutes in a 5% sodium hypochlorite solution.
The day after preparing the rhizophores, plantation was carried out in polyethylene bags (height = 22 cm, diameter = 15 cm, diameter = 15 cm, volumetric capacity = 1.5 liters) filled with substrate, composed by soil and manure. The analysis revealed 100 mg/dm3 of P, 657 mg/dm3 of K, 4.74 cmolc/dm3 of Ca, 0.97 cmolc/dm3 of Mg 3.05 cmolc/dm3 of H + Al, 7.67 g/kg of organic mater. The seedlings were kept under a shade netting (with 50% luminosity restriction), and manually irrigated twice a day (capacity/volume of 13 liters). Sixty days after planting, the following morphological characteristics were evaluated/measured: plant height, stem diameter, number of leaves, Dickson quality index (DQI), and leaf area (LA).
LA was estimated according to the model of indirect determination (LA LW= (−27.7418 + (3.9812 LW/ln LW) proposed by Erlacher et al. (2016) by using nondestructive measurements of leaf length (L) and/or width (W). Shoot diameter was measured using a digital caliper. Dry biomass was obtained with a forced circulation air oven at 65°C until stable weight. Biomass was determined using a digital scale.
The Dickson quality index (DQI) was determined by the formula (6):
DQI = [total dry mass (g) / (RHD + RSR)]
Total dry matter/weight = leaves dry matter/weight + stem dry matter/weight + tuberous root dry matter/weight + rhizophores dry matter /weight; RSR: relation between the dry material shoot matter/weight and tuberous root dry material; RHD: Relation between shoot height and stem diameter.
Physiological assesments were carried out through an infrared gas analyzer (IRGA Licor 6400XT). Estimations of net carbon assimilation rate (A), stomatal conductance (gs), leaf transpiration rate (E), water use efficiency (WUE), internal CO2 concentration (Ci), and instantaneous carboxylation efficiency were obtained (A/Ci ). Sixty days after transplanting (DAT), evaluations were carried out in a clear sky day, between 8 and 11 am, using standard fully developed leaves without visual occurrence of any anomalies (on the first stem that emerged). Photosynthetically active radiation was standardized under an artificial saturating light of 1000 μmol photons m-2 s-1 and the CO2 concentration in the chamber (420 ppm).
Determination of relative foliar chlorophyll a and b, and total content was carried out using a digital chlorophyll meter. The ClorofiLOG chlorophyll meter (CFL 1030 - Falker) uses emitting photodiodes in three wavelengths: two emit within the red band, close to the peaks of each type of chlorophyll (λ=635 and 660 nm), and the other emits in near-infrared (λ=880 nm). From these data, the device provides a value called the Falker chlorophyll index (FCI) that is proportional to the absorbance of both chlorophylls (2).
Data were submitted to ANOVA by the F-test . Means were compared by the Tukey’s test (p<0.05), using R software (26).
Development and field production
To track plant development until harvest, an experiment was conducted from April to October 2017 in the experimental area of the CCAE/UFES, in the municipality of Alegre-ES (20°45’ S and 41°29’ W, 113 m a. s. l.). This is a lowland region, in the Itapemirim River area, characterized as a warm tropical microregion (lowlands), with higher temperatures (22). The average monthly temperature ranged from 22°C to 26°C, and rainfall reached 201.6 mm during the experiment. The soil was classified as Red-Yellow Latosol of medium texture (7). A sample, collected 0-20 cm deep, was analyzed by the Soil Laboratory of CCAE/UFES, and presented: pH (water) = 5.73; Phosphorus Mehlich 1 (mg/dm3) = 34.79; Potassium (mg/dm3) = 42; Calcium (cmolc/dm3) = 2.51; Magnesium (cmolc/dm3) = 1.38; Aluminum (cmolc/dm3) = 0,00; Bases sum (cmolc/dm3) = 2,36; effective CTC (cmolc/dm3) = 2.36; Bases saturation (%) = 57.34; Total organic carbon (%) = 1; Total Nitrogen (%) = 0.1; Sand (%) = 55; Silt (%) = 4; Clay (%) = 30.
The experiment was carried out in a randomized complete block design with four replications. The treatments consisted of the seedlings produced in the nursery from rhizophores with different number of buds: one to two buds (T1); three to four buds (T2); five to six buds (T3); and seven to eight buds (T4). Each experimental plot consisted of 4 planting lines with 5 plants spaced 1.0 m between rows and 0.5 m between plants. The two central lines were considered the useful area, except for the border plants of each row.
Fertilization was performed by applying a cover of 180 g of prepared bovine manure per plant containing the following nutrients: 15.05 g kg-1 of N; 6.00 g kg-1 of P; 30.07 g kg-1 of K; 9.10 g kg-1 of Ca; and 8.75 g kg-1 of Mg. Throughout the cultivation cycle, manual control of spontaneous weeds was performed, as well as irrigation through conventional sprinkling, complementing rainfall during the cultivation cycle, in order to achieve 800 mm needed, according to Grau and Rea (1997).
During the vegetative cycle (60 to 220 DAT), monthly evaluations of morphological and physiological development were carried out: leaf area (LA), number of leaves, plant height (highest stem), number of stems per plant and stem diameter. Chlorophyll a and b as well as total leaf chlorophyll between 8 and 11 am, were also assessed. At 220 DAT, (A), (gs), (E), (WUE), (Ci), and (A/Ci ) were estimated. These evaluations were performed by an infrared gas analyzer (IRGA Licor 6400XT).
At that moment (220 DAT), capacity for dry mass accumulation (leaves, rhizophores, tuberous roots) and productivity of fresh tuberous roots (t ha-1) were evaluated and classified as high (length greater than 20 cm, diameter greater than 7 cm, and weight greater than 300 g), medium (length between 12 to 20 cm, diameter between 5 and 7 cm, and weight between 120 and 300 g), low (length less than 12 cm, diameter less than 5 cm and weight less than 120 g), and total (31).
Data were submitted to ANOVA by the F-test, and means were compared through Tukey’s test (p<0.05). In addition, regressions for the variables throughout time, were adjusted. The statistical analysis was performed with the open code software R (26).
RESULTS AND DISCUSSION
Nursery development
Seedlings from rhizophores with three to four buds (T2) were the ones showing the best development in relation to the other treatments, (T1), (T3) and (T4), presenting larger LA, stem diameter, number of leaves, shoot and root dry mass, and DQI. This was observed despite the statistical similarity between LA, number of leaves, and the shorter plant height, when compared to seedlings from rhizophores with five to six buds (T3) (table 1).
Table 1. Leaf area, plant height, stem diameter, number of leaves, dry mass in shoot and root, and Dickson quality index in yacon seedlings from rhizophores with different bud numbers, developed in nursery.
Tabla 1. Área foliar, altura de la planta, diámetro del tallo, número de hojas, masa seca en el brote y la raíz, y el índice de calidad de Dickson en plántulas de yacón de rizóforos con diferentes números de yemas, desarrollado en vivero.

Presenting higher LA and number of leaves, like seedlings from rhizophores with three to four (T2) and five to six buds (T3), shows the capacity of these seedlings to intercept light and convert it into stored chemical energy (photoassimilates), which will determine a successful initial development and plant growth. In relation to (T4), the decrease in LA and plant height could be due to the lower ability to intercept light and convert it into chemical energy, affecting growth and development of these plants. This capacity is also reflected in shoot and root dry mass accumulation, in which T2 seedlings resulted more efficient. This shoot dry biomass gain is also reflected in a bigger stem diameter, which may also be a good indicator of seedling quality, especially for field growth and survival, due to a higher capacity for root development (37). Increasing root dry mass may indicate increased absorption of water and nutrients, resulting in higher plant development and, consequently, higher metabolic efficiency. In addition, according to Rós et al. (2013) and Neumnn et al. (2017), roots with adequate development are more capable of bearing environmental changes, since the plants’ root system allows for better contact with soil.
As a consequence of a greater dry mass accumulation in shoot and roots, T2 seedlings present better DQI, indicating good development in nurseries, and a probable better development in the field (28). Overall, the DQI values observed were above 0.20, T2 seedlings showed DQI = 0.47 corresponding to high quality seedlings. However, this parameter may vary depending on handling, substrate type , pot volume, age of the evaluated seedling and, mainly, on the studied species (25).
Regarding photosynthetic pigments, higher values of a, b and total chlorophyll in T2 seedlings (rhizophores with three to four buds) were also observed (table 2, page 57).
Table 2. Relative chlorophyll (FCI - Falker chlorophyll index) a, b and total chlorophyll content in yacon seedlings from rhizophores with different bud numbers, developed in the nursery.
Tabla 2. Clorofila relativa (FCI - índice de clorofila Falker) a, b y total de clorofila en plántulas de yacon de rizóforos con diferentes números de yemas, desarrollado en vivero.
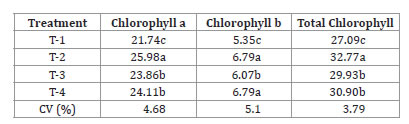
T-1= 1-2 buds; T-2 = 3-4 buds; T-3 = 5-6 buds; T-4= 7-8 buds. Averages in the column followed by the same letter are equal in the Tukey’s test, at a 5% probability.
T-1 = 1-2 yemas; T-2 = 3-4 yemas; T-3 = 5-6 yemas; T-4 = 7-8 yemas, los promedios en la columna seguidos por la misma letra son iguales en la prueba de Tukey, con una probabilidad del 5%.
They presented higher values both for chlorophyll a, which is the pigment used to perform the first stage of the photosynthetic process (photochemical reactions), and for chlorophyll b, pigments that help light absorption and the transference of radiant energy to reaction centers, being thus called “accessory pigments” (18, 35, 37). These results may indicate differences in photosynthetic capacity among seedlings, especially for T2 seedlings.
Concerning gas exchange, we observed that T2 and T3 seedlings presented the highest gs, which may have reflected on a higher E. and the highest A (table 3).
Table 3. Stomatal conductance, transpiration, net CO2 assimilation rate, water use efficiency, internal CO2 concentration and instantaneous carboxylation efficiency in leaves of yacon seedlings, from rhizophores with different bud numbers, developed in the nursery.
Tabla 3. Conductancia estomática, transpiración, tasa neta de asimilación de CO2, eficiencia en el uso del agua, concentración interna de CO2 y eficiencia de la carboxilación instantánea, en hojas de plántulas de yacón, de rizóforos con diferentes números de yemas, desarrolladas en vivero.

These seedlings presented the lowest (WUE) (table 3), meaning a lower amount of carbon fixated per each unit of transpired water, probably given by abundant irrigation and a bigger LA, and thus, a higher E. Growth of these seedlings presented higher dry mass accumulation. Higher Ci observed for leaves of T3 seedlings, were due to their higher gs, (table 3).
As for the efficiency of instantaneous carboxylation (A/Ci), the results were very close, although higher for T2 and T3. We conclude that the seedlings produced from rhizophores with three to four buds (T2) had better performances, proving to be the best option for the propagation of yacon.
Field development and production
Seedlings in the field presented similar data for plant height, stem diameter, number of stems and leaves per plant (figure 2, page 59).
Figure 2. Plant height (a), steam diameter (b), number of stems (c) and number of leaves (d) in yacon plants from rhizophores with different bud number, during the vegetative cycle.
Figura 2. Altura de la planta (a), diámetro del tallo (b), número de tallos (c) y número de hojas (d) en plantas de yacón de rizóforos con diferentes números de yemas, durante el ciclo vegetativo.
As for LA, differences, concerning the highest values for T2 seedlings (from rhizophores with three to four buds) in the period from 105 to 160 days after transplantation (DAT, figure 1), were observed.
Figure 1. Leaf area (a), relative chlorophyll (FCI - Falker chlorophyll index) a (c), b (d) and total chlorophyll (b) content in yacon plants from rhizophores with different bud number, during the vegetative cycle.
Figura 1. Área foliar (a), clorofila relativa (FCI - índice de clorofila Falker) a (c), b (d) y contenido total de clorofila (b) en plantas de yacón de rizóforos con diferentes números de yemas, durante el ciclo vegetativo.
This is an important observation, considering that, in this phase, yacon plants have a peak on the accumulation of reserves in tuberous roots, as observed by Silva et al. (2018a). LA is an indicator of photosynthetic potential, and these plants showed higher accumulation in reserve organs. Sugars obtained from the photosynthetic process are transported from the foliar limb to petioles and later to tuberous roots, where reserve carbohydrates are synthesized (13).
In accordance with this result, we also observed higher peaks of total chlorophyll contents in T2 plants, at 105 to 160 DAT, indicating that these plants could have been in better physiological conditions, as observed for the seedlings, presenting better capacity for performing photosynthesis. The total chlorophyll contents found represented chlorophyll a and b contents in leaves at those moments (105 to 160 DAT) (figure 1). As mentioned in the Introduction, higher chlorophyll a content is related to better light absorption, while higher amount of chlorophyll b is related to a higher efficiency (35). Estimations of chlorophyll a and b for T2 plants (figure 1) correlated with their A, showing a direct relation between these variables, and allowing for chlorophyll a and b contents to be effective indicators of the plant’s assimilation capacity.
These results are in agreement with Evans et al. (2017), Guan et al. (201 6) and Meschede et al. (2011), demonstrating the possibility of estimating the CO2 assimilation in yacon plants through chlorophyll contents, determined by “ClorofiLOG” (model FL1030, Falker), a device that has lower cost and simple handling than other photosynthesis analyzers, like IRGA Licor 6400XT, representing a significant methodological contribution.
Observing further physiological parameters, we found that T2 plants had higher E and A, as well as higher WUE, and (A/Ci) (table 4, page 60).
Table 4. Stomatal conductance, transpiration, net CO2 assimilation rate, water use efficiency, internal CO2 concentration, and instantaneous carboxylation efficiency in leaves of yacon, from rhizophores with different bud numbers, 220 days after transplantation.
Tabla 4. Conductancia estomática, transpiración, tasa neta de asimilación de CO2, eficiencia en el uso del agua, concentración interna de CO2 y eficiencia instantánea de la carboxilación en hojas de en plantas de yacón, de rizóforos con diferentes números de yemas, 220 días después del trasplante.
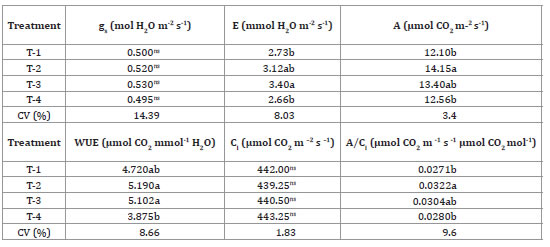
T-1= 1-2 buds; T-2 = 3-4 buds; T-3 = 5-6 buds; T-4= 7-8 buds. Averages in the column followed by the same letter are equal in the Tukey’s test, at a 5% probability. Stomatal conductance (gs), transpiration (E), net CO2 assimilation rate (A), efficiency in water use (WUE), internal CO2 concentration (Ci) and instantaneous carboxylation efficiency (A/Ci).
T-1 = 1-2 yemas; T-2 = 3-4 yemas; T-3 = 5-6 yemas; T-4 = 7-8 yemas, los promedios en la columna seguidos por la misma letra son iguales en la prueba de Tukey, con una probabilidad del 5%. Conductancia estomática (gs), transpiración (E), tasa neta de asimilación de CO2 (A), eficiencia en el uso del agua (EUA), concentración interna de CO2 (Ci) y eficiencia de carboxilación instantánea (A / Ci).
These results show that T2 plants (produced from rhizophores with three to four buds) have the capacity of incrementing the production of photoassimilates, due to the higher A probably caused by higher E, and instantaneous carboxylation efficiency (A/Ci), given that these two variables are directly related to A. We observed no significant differences among the treatments for gs. However, the gs values observed are equivalent to the ones obtained for C3 plants (37), indicating no limitation for CO2 for photosynthesis.
T2 plants had the highest accumulation of shoot and tuberous roots dry mass (table 5, page 60), followed by the second highest accumulated mass of rhizophores, showing that the investment in this organ was just enough for it to connect and transport photoassimilates from leaves to roots, and to constitute a propagation organ.
Table 5. Dry mass of shoot, rhizophores and tuberous roots of yacon plants, from rhizophores with different bud numbers, 220 days after transplantation.
Tabla 5. Masa seca en brotes, cepa y raíces tuberosas en plantas de yacón, de rizóforos con diferentes números de brotes, 220 días después del trasplante.
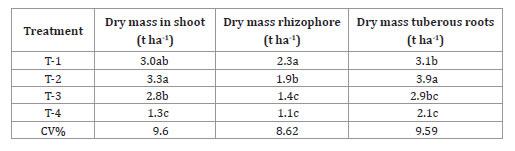
T-1= 1-2 buds; T-2 = 3-4 buds; T-3 = 5-6 buds; T-4= 7-8, Averages in the column followed by the same letter are equal in the Tukey test, at a 5% probability.
T-1 = 1-2 yemas; T-2 = 3-4 yemas; T-3 = 5-6 yemas; T-4 = 7-8, los promedios en la columna seguidos por la misma letra son iguales en la prueba de Tukey, con una probabilidad del 5%.
Plants seem to invest in this organ, since, according to Amaya (2002), the complete formation of rhizophores happens after photoassimilates arrive to tuberous roots, which will later work as a source of assimilates for rhizophores to be used as a survival strategy. T2 plants presented the highest productivity in fresh tuberous roots (table 6, page 60), which is the main way of commercializing this crop.
Table 6. Productivity, per class and total, of tuberous roots of yacon, from rhizophores with different bud numbers, 220 days after transplantation.
Tabla 6. Productividad, por clase y total, de raíces tuberosas de yacón, de rizóforos con diferentes números de brotes, 220 días después del trasplante.
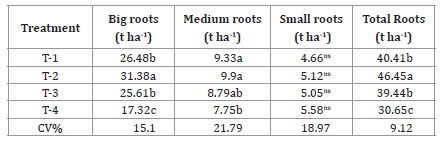
T-1= 1-2 buds; T-2 = 3-4 buds; T-3 = 5-6 buds; T-4= 7-8 buds. Averages in the column followed by the same letter are equal in the Tukey’s test, at a 5% probability, ns Non-significant. Classes proposed by Seminario et al. (2003).
T-1 = 1-2 yemas; T-2 = 3-4 yemas; T-3 = 5-6 yemas; T-4 = 7-86 yemas, los promedios en la columna seguidos por la misma letra son iguales en la prueba de Tukey, con una probabilidad del 5%, ns No significativo. Clases propuestas por Seminario et al. (2003).
Yacon also presented great variation in tuberous roots biomass given environmental conditions (weather and soil), crop handling (among which is the propagation material), and genetic variability (36). However, the observed productivity (30 to 40 t ha-1) is close to the highest productivity reported by Silva et al. (2018b) (31.7 t ha-1) in the same region, with plants propagated by stem cuttings.
To mention the influence of propagation on the categories of roots produced is important, as the financial feedback is related to this result (27). For this item, T2 plants stood out again, since they presented 67.63% of big roots productivity (31.38 t ha-1) and 21.33% of medium roots (9.9 t ha-1) (table 6, page 60), which are the root classes with the best commercial values.
Thus, our study shows that the number of buds in the yacon propagule (rhizophores) has direct influence, from seedling phase to final production, and that their weight should not be a factor for selection, given that propagules with 30 grams (T2 - with three to four buds) resulted even better than propagules weighing about 100 g.
Considering plant behavior, from seedling phase to the field, plants from rhizophores with three to four buds, resulted to be the best propagation form for the cultivation of yacon. The results suggest that these T2 plants, which had a good performance in the seedling phase, can keep a good vegetative development in the field. However, and even though T1 plants (from rhizophores with one to two buds), did not show such interesting results during seedling phase, they did present good vegetative development in the field.
CONCLUSIONS
Plants from rhizophores with three to four buds (about 30 grams) presented the best performance from seedling phase until final production, being an adequate propagation form for the cultivation of yacon.
1. Amaya, R. J. E. 2002. Desenvolvimento de “yacon” (Polymnia sonchifolia Poep. & Endl.) a partir de rizóforos e de gemas axilares, em diferentes espaçamentos. Tese de Doutorado. Universidade Estadual Paulista “Julio de Mesquita Filho. Botucatu, SP- Brasil. 89 p.
2. Barbieri, J. É; Rossiello, R. O. P; Silva, R. V. M. M; Ribeiro, R. C; Morenz, M. J. 2012. Um novo clorofilômetro para estimar os teores de clorofila em folhas do capim Tifton 85. Ciência Rural.42:2242-2245. doi: 10.1590/s0103- 84782012005000109 [ Links ]
3. Caetano, B. F.; Moura, N. A.; Almeida, A. P.; Dias, M. C.; Sivieri, K.; Barbisan, L. F. 2016. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients. 8: 436-440. http://dx.doi.org/10.3390/nu8070436 [ Links ]
4. Cocato, M. L; Lobo, A. R.; Azevedo. Martins, A. K.; Mancini F, J.; Sá, L. R. M.; Colli, C. 2019. Effects of a moderate iron overload and its interaction with yacon flour, and/or phytate, in the diet on liver antioxidant enzymes and hepatocyte apoptosis in rats. Food Chemistry. 285: 171-179. http://dx.doi.org/10.1016/j.foodchem.2019.01.142. [ Links ]
5. Coelho, M. D. F. B.; Azevedo, R. A. B. 2016. Efeito do tipo de estaca na propagação de Turnera subulata. Horticultura Brasileira. 34(3): 435-438. http://dx.doi.org/10.1590/s0102-05362016003021. [ Links ]
6. Dickson, A; Leaf, A. L.; Hosner, J. F. 1960. Quality appraisal of white spruce and white pine seedling stock in nurseries. The Forestry Chronicle. 36: 10-13. Available in: http://dx.doi. org/10.5558/tfc36010-1. [ Links ]
7. Embrapa - Empresa Brasileira de Pesquisa Agropecuária. 2014. Centro Nacional de Pesquisa de Solos. Sistema brasileiro de classificação de solos. Rio de Janeiro: Embrapa Solos. 92 p. [ Links ]
8. Erlacher, W. A.; Oliveira, F. L.; Fialho, G. S.; Silva, D. M. N.; Carvalho, A. H. O. 2016. Models for estimating leaf area GuanGuaof yacon. Horticultura Brasileira. 34(3): 422-427. http://dx.doi. org/10.1590/S0102-05362016003019. [ Links ]
9. Evans, J. R.; Morgan, P. B; Von C. S. 2017. Light quality affects chloroplast electron transport rates estimated from Chl fluorescence measurements. Plant and Cell Physiology. 58(10): 1652-1660. http://dx.doi.org/10.1093/pcp/pcx103. [ Links ]
10. Gomes, H. E.; Heredia, Z. N. A.; Vieira, M. C.; Pereira, G. R.; Pacito, T. E.; Vargas, M. R. 2010. Produção de mudas e de raízes comerciais de mandioquinha-salsa ‘Amarela de Carandaí’em função de espaçamentos e amontoa. Semina: Ciências Agrárias. 31(1): 1121-1132.
11. Grau, A.; Rea, J. 1997. Yacon Smallanthus sonchifolius Poepp. & Endl. In: Hermann, M. and Heller J. (Eds.). Andean roots and tubers: Ahipa, arracacha, maca and yacon. Promoting the conservation and use of underutilized and neglected crops. Institute of Plant Genetics and Crop Plant Research, Gatersleben/ International Plant Genetic Resources Institute, Rome. 198-242. [ Links ]
12. Guan, K.; Berry, J. A.; Zhang, Y.; Joiner, J; Guanter, L.; Badgley, G.; Lobell, D. B. 2016. Improving the monitoring of crop productivity using spaceborne solar-induced fluorescence. Global change biology. 22(2): 716-726. http://dx.doi.org/10.1111/gcb.13136. [ Links ]
13. Gusso, A. P.; Mattanna, P.; Richards, N. 2015.Yacon: benefíc Meschedeios à saúde e aplicações tecnológicas. Ciência Rural. 45(5): 912-919. http://dx.doi.org/10.1590/0103-8478cr20140963. [ Links ]
14. Hartmann, H. T.; Kester, D. E.; Davies, F. T. Jr.; Geneve, R. L. 2011. Plant propagation: principles and practices. 8th ed. Boston: Prentice-Hall. 915 p. [ Links ]
15. Ibañez, M. S.; Mercado, M. I.; Aráoz, M. C.; Zannier, M. L.; Grau, A.; Ponessa, G. I. 2017. Flower structure and developmental stages of the capitulum of Smallanthus sonchifolius (Asteraceae): reproductive implications. Journal of Plant Research. 130(2): 327-337. http://dx.doi. org/10.1007/s10265-017-0904-x. [ Links ]
16. Lorenzoni, R. M.; Menine, F.; Marques, J. E; Oliveira, F. l; Soares, T. C. B. 2017. Genetic diversity of yacon accessions using ISSR markers. Genetics and Molecular Research. 16: 1-8. http://dx.doi.org/10.4238/gmr16029576. [ Links ]
17. Machado, E. C.; Schmidt, P. T.; Medina, C. L.; Ribeiro, R. V. 2005. Respostas da fotossíntese de três espécies de citros a fatores ambientais. Pesquisa agropecuária brasileira. 40(12): 1161-1170. [ Links ]
18. Meloni, D. A.; Gulotta, M. R.; Silva, D. M.; Arraiza, M. P. 2019. Effects of salt stress on germination, seedling growth, osmotic adjustment, and chlorophyll fluorescence in Prosopis alba G. Revista de la Facultad de Ciencias Agrarias. Mendoza. Argentina. 51(1): 69-78. [ Links ]
19. Meschede, D. K.; Velini, E. D.; Carbonari, C. A.; Silva, J. R. M. 2011. Alteração fisiológica da canadeaçúcar pela aplicação de Glyphosate e Sulfumeturon-Methyl. Planta Daninha. 29(2): 413-419. http://dx.doi.org/10.1590/s0100-83582011000200019.(2011) [ Links ]
20. Neumann, E. R., Resende, J. T. V.; Camargo, L. P.; Chagas, R. R.; Lima F, R. B. 2017. Produção de mudas de batata doce em ambiente protegido com aplicação de extrato de Ascophyllumnodosum. Horticultura Brasileira. 35(4): 490-498. http://dx.doi.org/10.1590/s0102- 053620170404. [ Links ]
21. Pereira, E. G.; Siqueira, S. A. I.; Souza, A. E.; Melo, N. M. J.; Souza, J. P. 2017. Distinct ecophysiological strategies of widespread and endemic species from the megadiverse Campo rupestre. Flora. 238: 79-86. https://doi.org/10.1016/j.flora.2017.02.006. [ Links ]
22. Pezzopane, J. E. M.; Castro, F. S.; Pezzopane, J. R. M.; Cecílio, R. A. Agrometeorologia: aplicações para o Espírito Santo. Vitória-ES. 178 p. [ Links ]
23. Pfennigwerth, A. A; Bailey, J. K; Schweitzer, J. A. 2017. Trait variation along elevation gradients in a dominant woody shrub is population-specific and driven by plasticity. AoB Plants. 9: 1-13. https://doi.org/10.1093/aobpla/plx027 [ Links ]
24. Pimentel, N.; Lencina, K. H.; Pedroso, M. F.; Somavilla, T. M.; Bisognin, D. A. 2017. Morphophysiological quality of yerba mate plantlets produced by mini-cuttings. Semina: Ciências Agrárias. 38(6): 3515-3525. http://dx.doi.org/10.5433/1679-0359.2017v38n6p3515. [ Links ]
25. Puiatti, M.; Katsumoto, R.; Pereira, F. H.; Barrella, T. P. 2003. Crescimento de plantas e produção de rizomas de taro ‘Chinês’ em função do tipo de muda. Horticultura Brasileira. 21(1): 110-115. http://dx.doi.org/10.1590/s0102-05362003000100023.
26. R Development Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. Austria. Available in: http://www.R-project. org (Accessed 0ctober 2017). [ Links ]
27. Ree Kim, Y.; Mascarini, L.; Lorenzo, G. A.; González, M. N. 2018. Planting density and its effect on plant height and rosette quality in ornamental cabbages for cutting. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 50(2): 49-64. [ Links ]
28. Rodrigues, L. A.; Araujo, M. T.; Silva, S. S.; Cyrino, E. A. 2016. Qualidade de mudas de Moringa cultivadas em substratos com fibra de coco verde e compostos orgânicos. Revista Ceres. 63(4): 545-552. http://dx.doi.org/10.1590/0034-737x201663040016. [ Links ]
29. Rós, A. B.; Araujo, H. S.; Narita, N. 2013. Uso de fertilizante de liberação lenta na produção de mudas de batata-doce em bandeja. Semina: Ciências Agrárias. 34(6): 2667-2669. http://dx.doi.org/10.5433/1679-0359.2013v34n6p2667. [ Links ]
30. Santos, W. M.; Junior, S. S.; Nolasco, F.; Silva, C R. A.; Silva, M. B.; Rodrigues, L. F. O. S. 2014. Produção de mudas de taioba em função do tipo e seccionamento de rizomas. Científica. 42(1): 74-84. http://dx.doi.org/10.15361/1984-5529.2014v42n1p74-79. [ Links ]
31. Seminario, J.; Valderrama, M.; Manrique, E. I. 2003. El yacon: fundamentos para el aprovechamiento de un recurso promisorio. Centro Internacional de la Papa (CIP), Universidad Nacional de Cajamarca, Agencia Suiza para el Desarrollo y la Cooperación (COSUDE). Lima. 61 p. [ Links ]
32. Siarovská, J.; Padilla. G. G. F; Viehmannová, I.; Fernández, E. 2019. Genetic and chemical diversity among yacon [Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson] accessions based on iPBS markers and metabolomic fingerprinting. Plant Physiology and Biochemistry. 141: 183-192. http://dx.doi.org/10.1016/j.plaphy.2019.05.0. [ Links ]
33. Silva, D. M. N. D.; Oliveira, F. L. D.; Cavatte, P. C.; Quaresma, M. A. L.; Christo, B. F. 2018a. Growth and development of yacon in different periods of planting and growing regions. Acta Scientiarum Agronomy. 40(1): 394-402. http://dx.doi.org/10.4025/actasciagron.v40i1.39442. [ Links ]
34. Silva, D. M. N.; Venturim, C. H. P.; Capucho, M. E. O. V.; Oliveira, F. L.; Mendonça, S. E. 2018b. Impact of soil cover systems on soil quality and organic production of yacon. Scientia Horticulturae. 235: 407-412. http://dx.doi.org/10.1016/j.scienta.2018.03.024. [ Links ]
35. Streit, N. M.; Canterle, L. P.; Canto, M. W. D.; Hecktheuer, L. H. H. 2005. The chlorophylls. Ciência Rural. 35(3): 748-755. [ Links ]
36. Sumiyanto, J.; Dayan, F. E.; Cerdeira, A. L.; Wang, Y. H.; Khan, I. A.; Moraes, R. M. 2012. Oligofructans content and yield of yacon (Smallanthus sonchifolius) cultivated in Mississippi. Scientia Horticulturae. 148: 83-88. http://dx.doi.org/10.1016/j.scienta.2012.09.020. [ Links ]
37. Taiz, L.; Zeiger, E.; Moller, I. M; Murphy, A. 2017. Fisiologia e desenvolvimento vegetal. 6º ed. Porto Alegre. Artmed. 649 p. [ Links ]
38. Thomas, F. M.; Yu, R.; Schäfer, P.; Zhang, X.; Lang, P. 2017. How diverse are Populus “diversifolia” leaves? Linking leaf morphology to ecophysiological and stand variables along water supply and salinity gradients. https://doi.org/10. 1016/j.flora.2017.05.007
39. Torales, E. P.; Heid, D. M.; Abrão, M. S.; Zárate, N. A. H.; Carmo, V. M.; Santos, C. C.; Aran, H. D. V. R. 2019. Produção agroeconômica de Xanthosoma mafaffa Schott sob diferentes tamanhos de mudas e bases de cama de frango. Revista de Ciências Agrárias. 42: 648-656. https://doi.org/10.19084/rca.17571. [ Links ]
40. Torres, L. F.; Cavatte, P. C.; Garbin, M. L.; Hollunder, R. K.; Ferreira, S. K.; Capetine, T. B.; Soares, B. S.; Carrijo, T. T. 2019. Surviving in the shadows: light responses of co-occurring rubiaceae species within a tropical forest understory: Light responses of co-occurring Rubiaceae species within a tropical forest understory. Flora. 261:1-13. http://dx.doi.org/10.1016/j. flora.2019.151487. [ Links ]
41. Valladares, F.; Niinemets, Ü., 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 39: 237-257. https://doi.org/10.1146/annurev. ecolsys.39.110707.173506. [ Links ]
42. Vilhena, S. M. C.; Câmara, F. L.; Kakihara, S. T. 2000. O cultivo de yacon no Brasil. Horticultura Brasileira. 18(1): 5-8. http://dx.doi.org/10.1590/s0102-05362000000100002 [ Links ]
43. Zárate, N. A. H.; Vieira, M. C. 2005. Produção da araruta ‘Comum’ proveniente de três tipos de propágulos. Ciência e Agrotecnologia. 29: 995-1000. http://dx.doi.org/10.1590/s1413- 70542005000500012.
ACKNOWLEDGEMENTS
The authors would like to thank Fundação de Amparo à Pesquisa do Espírito Santo (FAPES, Vitória-ES, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília-DF, Brazil) for their financial support and granting scholarships, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília-DF, Brazil) for the scholarship to the first author.














