Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
Print version ISSN 1853-8665On-line version ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.52 no.2 Mendoza Dec. 2020
ORIGINAL ARTICLE
Effects of a glycogenic additive on intake, daily gain, and blood metabolites in weaned horses
Efecto de un aditivo glucogénico sobre consumo, ganancia diaria y metabolitos sanguíneos en caballos destetados
Eduardo Morones 1, Paulina Pérez-Estrada 2, Germán Mendoza-Martínez 2, Abel Hernández-García 3, Antonio Martínez-García 2*
1 Unidad de Policía Metropolitana “UPN Montada”. Secretaría de Seguridad Ciudadana. Gobierno de la Ciudad de México. México 09209.
2 Universidad Autónoma Metropolitana Unidad Xochimilco. Licenciatura de Medicina Veterinaria y Zootecnia. Departamento de Producción Agrícola y Animal. Calzada del Hueso 1100. Col. Villa Quietud. Alcaldía Coyoacán. Ciudad de México. México. 04960. * jamgar@correo.xoc.uam.mx
3 Centro Universitario UAEM-Amecameca. Universidad Autónoma del Estado de México. México. 56900.
Originales: Recepción: 06/05/2019 - Aceptación: 07/07/2020
ABSTRACT
The objective of this experiment was to evaluate a glycogenic feed additive in terms of performance and response in blood metabolites in weaned horses. Twenty weaned horses of the Unidad de Policía Montada Metropolitana del Ministerio de Seguridad Pública del Gobierno de la Ciudad de México, México, were randomly assigned to two treatment groups: control or an oral dose of the glycogenic additive (10 g/d). The additive was based on propylene glycol and Na/Ca propionate and was supplied for 30 days. Data were tested for normality and initial weight was used as a covariate for analyzing the average daily gain (ADG). The ADG was negatively affected (P < 0.01) by the glycogenic compound (0.468 vs. 0.517 kg), while there were no significant differences (P > 0.10) in blood glucose, globulins, total glucose, triglycerides, cholesterol, creatinine or urea in horses from either treatment group. It was concluded that the glucogenic additive, based on propylene glycol and Na/Ca propionate, negatively affected the performance of the horses. Therefore, administration of the additive is not recommended in weaned horses.
Keywords: Creatinine; Glucose; Horses; Propylene glycol; Triglycerides
RESUMEN
El objetivo de este experimento consistió en evaluar un aditivo glucogénico en el rendimiento de los caballos destetados y en la respuesta de los metabolitos sanguíneos. Se utilizaron veinte caballos destetados de la Unidad de Policía Montada Metropolitana del Ministerio de Seguridad Pública del Gobierno de la Ciudad de México, México. Se asignaron al azar a dos tratamientos, control o dosis orales de aditivo glicogénico (10 g/d) donde se basó el aditivo propilenglicol y propionatos de Na/Ca, suministrados durante 30 días. Los datos se probaron para determinar su normalidad y el peso inicial se usó como covariable para los análisis de los datos de ganancia diaria. La ganancia diaria se vio afectada negativamente (P < 0,01) por el compuesto glucogénico (0,468 vs. 0,517 kg), y no hubo diferencias significativas (P > 0,10) en las concentraciones de glucosa en sangre, globulinas, glucosa total, triglicéridos, colesterol, creatinina. o urea de caballos de cualquier grupo de tratamiento. En este estudio se concluye que el aditivo glucogénico basado en propilenglicol y propionato Na/Ca afectó negativamente el rendimiento del caballo, por lo que no se recomienda su administración en caballos destetados.
Palabras clave: Creatinina; Glucosa; Caballos; Propilenglicol; Triglicéridos
INTRODUCTION
The cecum and colon of equidae have been considered to perform a similar function to the rumen regarding the metabolism of volatile fatty acids. Experiments with isotopes have indicated that about 7% of the total glucose produced is synthesized from propionate produced in the cecum (12). However, propionate can be produced from rapidly fermentable carbohydrates that can also yield lactate, potentially leading to a cascade of events culminating in laminitis (21).
Pregnant mares and post-weaning foals are often fed concentrates rich in soluble carbohydrates, together with forage. Recent studies suggest that the use of concentrates is linked to alterations of metabolism and the development of osteochondrosis in foals (23).
As an alternative to increasing glucose precursors, glycogenic substances have been provided in the diet of ruminants, including propylene glycol (5) and calcium or sodium propionate (3, 13). Some feed additives including glycogenic precursors are available for horses, however, the scientific information on their benefits is scarce. Some feed additives, including glycogenic precursors, are available for horses, however, scientific information on their benefits is scarce, there are published data on propylene glycol poisoning (9), and in growing foals it may affect sexual maturity and metabolism up to 24 months of age (23). Propylene glycol (PG) (1-2 propanediol) is a 3-carbon (C3 H8 O2) molecule derived from propylene. When this is fermented in the rumen or cecum, the acetate: propionate ratio is decreased, resulting in a more glycogenic pattern of volatile fatty acids, while the pH is generally not affected (18).
Therefore, the objective of this experiment was to evaluate the effects of a daily oral dose of a glycogenic product on performance and blood metabolites in weaned horses.
Objective
The objective of this experiment was to evaluate a glycogenic feed additive commercial (1,2-propanediol and Na/Ca propionate) on performance and blood metabolites in weaned horses, as an indicator for the reduction of grains in the diet.
MATERIAL AND METHODS
This research was conducted at the facilities of the Unidad de Policía Montada Metropolitana de la Secretaría de Seguridad Pública del Gobierno de la Ciudad de México, Mexico, which is located at an altitude of 2,247 m, North latitude of 19°21’30” and West longitude of 99°05’35”. The study was performed under the supervision of the Comité de Bioética y Bienestar Animal de la Corporación de la Policía Metropolitana de México and according to la Ley de Protección Animal del Distrito Federal, México (1).
Twenty weaned male Azteca horses (six months old) with initial weights ranging from 144.03 to 179.21 kg and an average weight of 164.35 ± 5.87 kg (μ ± standard error), were randomly allotted to the following two treatment groups: 1) control (10 ml distilled water) or 2) an oral dose of 10 g/d of glycogenic additive (LipoFeed PB®; Premezclas Energéticas Pecuarias S.A. de C.V., México) based on 1,2-propanediol and Na/Ca propionate in distilled water, which was dosed according to the manufacturer´s instructions after the first meal of the day. All horses where they were housed in individual stables, with feeder and access ad libitum to water, the water was not analyzed, because it is the same water that is supplied for human consumption.
The fed a ration (dry basis) composed of 20% commercial concentrate (Super Champion Horse® Nutrigafer S.A. de C.V., México), 60% oat straw and 20% alfalfa hay, with a nutrient content dry matter (DM) basis consisting of 12.6% crude protein (CP), 3.4% ether extract (EE), 30.4% neutral detergent fiber (NDF), and 16.3% acid detergent fiber (ADF).
At weaning the foals were weighed weekly during the first post-weaning month, initial weight (IW) and final weight (FW) were recorded to estimate live weight changes. Live weight was recorded using a balance with a capacity of 2 t in order to estimate the horses’ average daily gain. The blood concentrations of metabolites were determined from blood samples collected via jugular venipuncture 3 h after feeding on day 30 of the experiment. After collection, the blood samples were centrifuged at 1500 x g for 20 min. The blood samples were kept refrigerated during transport and sampling and were analyzed within the first 6 h after extraction. Blood glucose was measured using glucose oxidase, urea using urease, creatinine using picric acid, triglycerides using a four-step enzymatic procedure and cholesterol using cholesterol esterase/cholesterol oxidase (14).
To determine the volatile fatty acid profile of the feed additive, a sample of the glycogenic product (20 mg) was dissolved in methanol and subjected to gas chromatography (6890; Agilent, Santa Clara, CA) using propionic, acetic and butyric acids as standards (Sigma-Aldrich Canada, Oakville, ON, Canada). Samples of the glycogenic product were also analyzed for organic matter and ashes (2) and the gross energy was determined using an adiabatic bomb calorimeter (Model 1241; Parr Instrument Co., Moline, IL). Data were tested for normality and analyzed with JMP7 software (24). Initial weight was used as a covariate for analyses of daily gain data and blood metabolites (4).
The results were analyzed according to a completely randomized design with two treatments and the initial weight was used as a covariate (25).
RESULTS
Oral administration of the glycogenic additive, which was based on propylene glycol and Na/Ca propionates, for 30 days negatively affected (P < 0.01) daily gain in the weaned horses (table 1, page 392), while there were no significant differences in blood metabolites associated with energy metabolism between horses in the treatment and control groups (P > 0.10).
Table 1. Effect of daily dosing of the glucogenic additive (GA) on the performance and blood metabolites of weaned horses.
Tabla 1. Efecto del aditivo glucogénico (GA) dosificado diariamente sobre el rendimiento de caballos destetados y metabolitos sanguíneos.
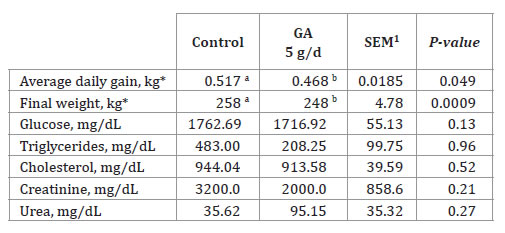
*Initial weight was used as a covariate (P < 0.05). a,b, Indicates that there is a significant difference between the two letters (P < 0.05). 1SEM: Standard error of the mean.
*Peso inicial utilizado como covariable (P < 0,05). a, b, Las medias con distintas letras son diferentes (P < 0,05). 1SEM: Error estándar de la media.
The product analyzed contained a high amount of ash (56.08 ± 0.56), low gross energy (1.316 ± 0.080 Mcal/kg) and low propionate concentration (21.767 mM).
DISCUSSION
The negative effects from the feed additive may be associated with its propylene glycol content (the product label indicates that it contains 3.3% 1,2-propanediol). Propylene glycol is metabolized to lactate in the liver, via hepatic alcohol and aldehyde dehydrogenases, which can then be used for gluconeogenesis (6), however, even when the concentration of propylene glycol in the blood is low, it may increase lactate in the blood. Non-fatal intoxications with propylene glycol have been reported from lower doses (8) but fatal toxicosis has been reported following the accidental oral administration of 6.0 ml propylene glycol/kg (15) or 3.8 L of propylene glycol (9). In our experiment, no signs of clinical toxicosis were observed in the horses, however, they did refuse oral drenches of the additive.
Although propylene glycol oral drenches have been shown to increase blood glucose concentrations in ruminants (5, 11), in the current study, the glucose and other blood metabolites remained unchanged. In horses, propylene glycol is metabolized in the liver, while in ruminants it is metabolized in the rumen through propionate. Additionally, Ca and Na propionate did not have any beneficial effects on the horses’ blood glucose concentrations. The addition of Ca propionate in vitro (17) or to lambs’ diets (16) has failed to increase propionate in rumen fermentation, therefore, it is unlikely to have an impact through cecal fermentation in horses. The additive did not demonstrate any glycemic effectiveness from the propionates or the propylene glycol.
The National Research Council (NRC,) for horses (19) has not recognized glycogenic compounds in feed. In contrast, it has presented a list of additives that improve animal health and performance, such as probiotics, enzymes, herbs and botanical oligosaccharides, yeast cultures and extracts that can affect cecal fermentation (10). Based on the measured energy, ash and propionate, the glycogenic additive did not contain the energy (not credible and biological values) and Na/Ca propionates reported on the data sheet from the manufacturer (approximately 54 mM equivalent of propionate). Concern has been expressed regarding the lack of regulation and the availability of some products that prevent or treat osteoarthritis in horses (7, 20, 22). These concerns may be extrapolated to products containing propylene glycol that should not been recommended as feed additives in equine nutrition.
CONCLUSION
It was concluded that the glycogenic additive under study, based on propylene glycol and Na/Ca propionate, negatively affected performance and should not be fed to horses.
1. ALDF. 2014. Law protecting animals Federal District, Mexico. Legislative Assembly of the Federal District, VII Legislature. Published in the Official Gazette of the Federal District, Mexico. February 26, 2002. Last reform published in the Official Gazette of the Federal District, Mexico on 18 December 2014. [ Links ]
2. AOAC. 1990 Official methods of analysis. 15th ed., Association of Official Analytical Chemists; Virginia USA: 1990. [ Links ]
3. Berthelot, V.; Bas, P.; Schmidely, P.; Duvaux-Pontes, C. 2001. Effect of dietary propionate on intake patterns and fatty acid composition of adipose tissues in lambs. Small Ruminant Research. 40: 29-39. doi.org/10.1016/S0921-4488(00)00217-0 [ Links ]
4. Celaya, R.; Ferreira, L. M. M.; García, U.; García, R. R.; Osoro, K. 2012. Heavy grazing by horses on healthlands of different botanical compositions. In: Forages and Grazing in Horse Nutrition. Eds. Saastamoinen M, Fradinho MJ, Santos AS, Miraglia N. 132: 219-226. doi.org/10.3920/978-90-8686-755-4_26 [ Links ]
5. Chiofalo, V.; Todaro, M.; Liotta, L.; Margiotta, S.; Manzo, T.; Leto, G. 2005. Effect of propylene glycol on pre- and postpartum performance by dairy ewes. Small Ruminant Research. 58: 107-114. doi.org/10.1016/j.smallrumres.2004.09.001 [ Links ]
6. Christopher, M. M.; Eckfeldt, J. H.; Eaton, J. W. 1990. Propylene glycol ingestion causes D-lactic acidosis. Lab Investigation. 62: 114-118. [ Links ]
7. Dechant, J. E.; Baxter, G. M. 2007. Glucosamine and chondroitin sulphate as structure modifying agents in horses. Equine Veterinary Education. 19: 90-96. doi.org/10.2746/095777307X180385 [ Links ]
8. Deprez, P.; Deconinck, R.; Lefère, L.; De Clercq, D. 2002. Propyleenglycol intoxicatie bij een pony (Propylene glycol intoxication in a pony). Vlaams Diergen Tijds. 71: 419-422. doi. org/10.1111/j.1476-4431.2011.00688.x [ Links ]
9. Dorman, D. C.; Haschek, W. M. 1991. Fatal propylene glycol toxicosis in a horse. Journal of the American Veterinary Medical Association. 198: 1643-1644. [ Links ]
10. Elghandour, M. Y. M.; Kholif, A. E.; Lopez, S.; Mendoza, G. D.; Odongo, N. E.; Salem, A. Z. M. 2016. In vitro gas, methane and carbon dioxide productions of high fibrous diet incubated with fecal inocula from horses fed live yeasts in response to the supplementation with different yeast additives. Journal of Equine Veterinary Science. 38: 64-71. doi.org/10.1016/j. jevs.2015.12.010 [ Links ]
11. Ferraro, S. M.; Mendoza, G. D.; Miranda, L. A.; Guitierrez, C. G. 2016. In vitro ruminal fermentation of glycerol, propylene glycol and molasses combined with forages and their effect on glucose and insulin blood plasma concentrations after an oral drench in sheep. Animal Feed Science and Technology. 213: 74–80. doi.org/10.1016/j.anifeedsci.2016.01.010
12. Ford, E. J. H.; Simmons, H. A. 1985. Gluconeogenesis from caecal propionate in the horse. British Journal of Nutrition. 53: 55-60. doi.org/10.1079/BJN19850010 [ Links ]
13. Lee, H.; Mendoza, G. D.; González, S. 2012. Effect of calcium propionate and sorghum level on lamb performance. Animal Feed Science and Technology. 177: 237-241. doi.org/10.1016/j. anifeedsci.2012.08.012 [ Links ]
14. Leidinger, E. F.; Leidinger, J.; Figl, J.; Rumpler, B.; Schwendenwein, I. 2015. Application of the ASVCP guidelines for the establishment of hematologic and biochemical reference intervals in Icelandic horses in Austria. Acta Veterinaria Scandinavica. 57: 30-40. doi.org/10.1186/ s13028-015-0120-4. [ Links ]
15. McClanahan, S.; Hunter, J.; Murphy, M.; Valberg, S. 1998. Propylene glycol toxicosis in a mare. Veterinary and Human Toxicology. 40: 294-296. [ Links ]
16. Mendoza, M. G. D.; Pinos-Rodríguez, J. M.; Lee-Rangel, H. A.; Hernández-García, P. A.; Rojo-Rubio, R.; Relling, F. A. 2015. Effects of dietary calcium propionate on growth performance and carcass characteristics of finishing lambs. Animal Production Science. 56: 1194-1198. doi. org/10.1071/AN14824. [ Links ]
17. Miranda, L. A.; Lee-Rangel, H. A.; Mendoza-Martínez, G. D.; Crosby-Galván, M. M.; Relling, A. E.; Pinos-Rodríguez, J. M.; Rojo Rubio, R.; González Hernandez, M. 2017. Influence of calcium propionate on in vitro fermentation of sorghum-based diets. Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo. Mendoza. Argentina. 49(1): 185-192. [ Links ]
18. Nielsen, N.; Ingvartsen, K. 2004. Propylene glicol for dairy cows: A review of the metabolism of propylene glycol and its effect of physiological parameters, feed intake, milk production and risk of ketosis. Animal Food Science Technology. 115: 191-213. [ Links ]
19. NRC. 2007. National Research Council. Nutrient Requirements of Horses. 6th ed. National Academy Press, Washington, D.C. p. 341. [ Links ]
20. Oke, S.; Aghazadeh-Habashi, A.; Weese, J. S.; Jamali, F. 2006. Evaluation of glucosamine levels in commercial equine oral supplements for joints. Equine Veterinary Journal. 38: 93-95. doi. org/10.2746/042516406775374306 [ Links ]
21. Pagan, J. D. 2008. Nutritional management of metabolic conditions. In: Advances in Equine. Nutrition IV. Ed: J. D. Pagan. Nottingham University Press. Nottingham United Kingdom. p. 269-276. [ Links ]
22. Ramey, D. W.; Eddington, N.; Thonar, E.; Lee, M. 2002. An analysis of glucosamine and chondroitin sulfate content in oral joint supplement products. Journal of Equine Veterinary Science. 22: 125-127. doi.org/10.1016/S0737-0806(02)70125-1. [ Links ]
23. Robles, M.; Gautier, C.; Mendoza L.; Peugnet, P.; Dubois, C.; Dahirel, M.; Lejeune, J-P.; Caudron, I.; Guenon, I.; Camous, S.; Tarrade A.; Wimel, L.; Serteyn D.; Bouraima-Lelong, H.; Chavatte Palmer, P. 2017. Maternal nutrition during pregnancy affects testicular and bone development, glucose metabolism and response to overnutrition in weaned horses up to two years. PLoS ONE 12: e0169295. doi:10.1371/journal.pone.0169295 [ Links ]
24. Sall, J.; Lehman, A.; Stephens, M.; Loring, S. 2012. JMP® Start Statistics: A Guide to Statistics and Data Analysis. SAS Institute Inc. Cary, North Caroline, USA. p. 28. [ Links ]
25. Steel, R. G. D.; Torrie, J. H. 1996. Bioestadística: Principios y Procedimientos. 2º Edición. McGrawHill. 622 p. [ Links ]














