Introduction
Euterpe edulis is a palm species, native to Brazil, that grows in the Northeastern, Midwestern, Southeastern and Southern regions, predominantly in the Cerrado and Atlantic Forest biomes 11. These biomes are part of 35 worldwide biodiversity hotspots, being a priority for the biodiversity conservation process 13.
Palm trees have suffered a predatory and, in most cases, illegal form of exploitation. Even though a sustainable plan is included in the Official List of Brazilian Species Threatened with Extinction since 2008, drastic reductions in its populations have been reported 14.
E. edulis, popularly known as Juçara, is a symbolic species of the Atlantic Forest with ecological, cultural, and economic value 6. Brazil outstands as the largest producer and exporter of canned hearts of palm worldwide. In addition to heart of palm, Juçara also produces rounded fruits, with bright black colored pulp covering the seeds that, after pulping, is freshly consumed or used for different types of drinks, ice cream, sweets and sauces 6. Palm fruit pulp has high nutritional value, with considerable content of proteins, sugars, fibers, and a lipid fraction with high content of polyunsaturated fatty acids, oleic acid, low content of saturated lipids and high content of phenolic compounds 4.
The sustainable exploitation of Juçara has been stimulated by pulp production, product with similar properties to Euterpe oleraceae Açaí pulp 23 has aroused the interest of the food industry, increasing the demand for seedlings, representing up to 73% of the costs of Juçara palm production 6. Studies on planting, management and seedling production techniques, as well as ecology and plant growth dynamics, are required for sustainable exploration and reintroduction of the species in its habitat 15.
Among seedling production techniques, inoculation with arbuscular mycorrhizal fungi (AMF) has proved strategic to produce Juçara palm seedlings in nurseries (Sgrott et al., 2012; Moreira et al., 2016). Inoculation of seedlings with mycorrhizal fungus usually results in rapid plant growth, with greater absorption of nutrients and enhanced tolerance to environmental stresses after field transplantation 16,17.
Although records have shown positive effects of mycorrhizal inoculation on Juçara palm seedlings, no study has yet shown the effect of phosphorus (P) doses on plant initial growth and nutritional status. However, AMF colonization and efficiency are influenced by the availability of this nutrient in the soil 23.
In this sense, Souza et al. (2011) suggested that studies aiming to obtain higher plant absorption efficiency and mycorrhizal symbiosis under low P availability, are essential to ensure sustainability of the Brazilian agribusiness. Thus, this work aimed to evaluate the effect of AMF inoculation on growth and nutrition of Juçara palm seedlings, with and without phosphate fertilization, verifying technical applicability in the production of Juçara palm seedlings.
Material and methods
Experimental location and substrate preparation
The experiment was conducted in a greenhouse at the Federal University of Espírito Santo, municipality of Alegre, State of Espírito Santo, Brazil, (20°47’1” S, 41°36’56” W, 640 m a. s. l.), between April and December 2018. Arbuscular mycorrhizal fungi (AMF) species Rhizophagus clarus Nicolson & Schenck and Claroideoglomus etunicatum Becker & Gerd, were obtained from the inoculum bank of the Soil Microbiology Sector - Laboratory of Soils, “Darcy Ribeiro” State University of Northern Rio de Janeiro (UENF), municipality of Campos dos Goytacazes-RJ, Brazil. Inocula were multiplied in association with Brachiaria brizantha for 90 days and grown in 5-kg capacity pots filled with previously sterilized soil.
For sowing, B. brizantha seeds were disinfected in 0.5% hypochlorite solution for 15 minutes and washed with deionized water four consecutive times. After 90 days, shoots were cut and pots were covered with kraft paper bags without irrigation for 30 days, facilitating fungi sporulation. Subsequently, soil mixture holding colonized roots and AMF spores was kept in cold chamber at 4°C until the experiment was conducted.
The substrate constituted a mixture of soil (collected at 0-20 cm depth, sieved in 2 mm mesh), and sand at 1: 2 ratio (v/v). Subsequently, this substrate was sterilized twice in autoclave (121°C for one hour), eliminating native fungi. After sterilization, chemical analysis of the substrate was carried out at the Laboratory of Soil Analysis, Federal Rural University of Rio de Janeiro (UFRRJ), campus of Campos dos Goytacazes-RJ. Table 1 shows substrate chemical characteristics.
Table 1: Tabla 1: Substrate chemical analysis after sterilization. Análisis químico del sustrato esterilizado.

P doses were applied with KH2PO4. Alternatively, Potassium (K) was increased to 113 mg dm-3 by supplying K2SO4 in treatments without P. After fertilization, the substrate was transferred to plastic bags and incubated for 45 days. After this period, P analysis was performed in all treatments with Mehlich-1 extractor, obtaining the following results: 6 and 36 mg dm-3 for P doses of 0 and 50 mg dm-3 respectively.
Inoculum preparation, inoculation and seed germination
E. edulis seeds were obtained from the processing of Juçara palm fruits, from the Pedro Menegardo Bortolotti industry located at São Vicente, rural area of Rio Novo do Sul-ES, Brazil.
On 05/22/2018, three seeds were sown at approximately one centimeter depth. Concomitantly, in treatments having AMF, 30 cm3 bag-1 were inoculated, at approximately 5 cm depth. Control treatment received no inoculation.
The experiment followed a randomized block design, 2x4 factorial arrangement and four replicates, three microbiological treatments with Rhizophagus clarus and Claroideoglomus etunicatum, mixed inoculum (Rhizophagus clarus + Claroideoglomus etunicatum) and control without AMF, in the absence and presence of P (50 mg kg-1).
According to greenhouse temperature and humidity conditions, daily irrigations maintained almost constant substrate moisture. At 102 days after sowing, thinning was performed, leaving two seedlings per seedling bag. Concomitantly, at 81, 116 and 151 days after sowing, 20 mg dm-3 nitrogen (N), as ammonium nitrate (NH4NO3) was applied to all treatments. Later, at 171, 185 and 203 days after sowing, Hoagland and Arnon (1950) nutrient solution without P, was also added.
Chemical, biometric and quality analysis of seedlings
At 226 days after sowing, concluding the experiment, leaf area (LA) was measured with a leaf area meter (model LI-3100 LICOR, Lincoln, NE, USA). Subsequently, shoots were packed in paper bags and placed in a forced-air ventilation oven at 70°C for 7 days until constant mass. Then, dry shoot mass (DSM) was determined using a digital scale (± 0.001)). Later, the material was crushed in a Wiley mill (20 mesh sieve), and hermetically packed in sealed plastic tubes. Finally, macronutrient levels were determined by chemical analysis.
N was determined after sulfuric digestion, by the method of Nessler 5. P, K, calcium (Ca), magnesium (Mg) and sulfur (S) levels were determined using Shimadzu® plasma (ICPE-9000) after digestion with HNO3 and H2O2, in an open digestion system 17. Nutrient contents were calculated by multiplying nutrient concentrations by the respective dry shoot mass.
Fragments of the finest roots were collected and stored in 50% ethyl alcohol for later determination of mycorrhizal colonization percentage according to Koske and Gemma (1989), and adapted by Sgrott et al. (2012), since according to these authors, the original method resulted in dark roots impairing proper visualization of fungal structures. The method was applied as follows: 1) roots were immersed in 10% KOH solution for 72 hours (solution change every 24 hours); 2) roots immersed in KOH were placed in water bath at 90°C for 60 minutes; 3) roots were washed under tap water and immersed in alkaline H2O2 solution for 30 minutes; 4) after washing, roots were placed in 1% HCl solution for 20 minutes; 5) After removing HCl, roots were covered with 0.05% trypan blue solution and left for another 60 minutes in water bath (90°C); 6) roots were kept in distilled water at 4°C until analysis. To verify root colonization, ten 10 segments of thin roots of approximately 1 cm were placed by tweezers after staining on the slide. A few drops of PVLG (polyvinyl-lacto-glycerol) were added and covered with cover slip. Then, samples were observed with a 100x optical microscope, evaluating fungal structures (hyphae, arbuscules and vesicles) based on Giovanetti and Moose (1980).
Data obtained were compared by ANOVA, and Tukey test at 5%, using the SANEST software 27.
Results and discussion
Mycorrhizal colonization percentage
Mycorrhizal colonization (%) of Juçara palm roots was significantly affected by fungus and P doses. Colonization ranged from 18.33% for treatment with C. etunicatum at 50 mg dm-3 P, to 58.33% for treatment with R. clarus at 0 mg dm-3 P. Mycorrhizal colonization was not detected in control treatments, assuring any undesired contamination (Table 2).
Table 2: Tabla 2: Mycorrhizal colonization percentage of Juçara palm seedlings as a function of arbuscular mycorrhizal fungi (AMF) and phosphorus (P) content at 226 days after sowing. Porcentaje de colonización micorrízica de plántulas de palma Juçara en función de hongos micorrízicos arbusculares (AMF) y fertilización con fósforo 226 días después de la siembra.
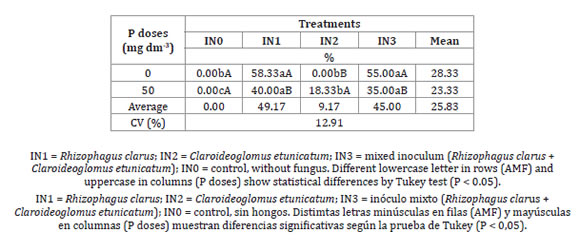
At 0 mg dm-3 P, no mycorrhizal colonization occurred with C. etunicatum, suggesting that this isolate causes no infection in Juçara palm roots under low P availability. In fact, C. etunicatum structures were only detected under 50 mg dm-3 P (Table 2, page 112). On the contrary, treatments with R. clarus and mixed inoculum showed higher colonization rates without phosphate fertilization and lower colonization when P was added to the soil. Decreasing mycorrhizal colonization percentage with increased P is normal, and, in many cases related to plant nutritional status 3.
Previously, Sgrott et al. (2012) and Moreira et al. (2016) had reported difficulty in quantifying mycorrhizal colonization in Juçara palm seedlings due to the anatomical characteristics of E. edulis roots. Moreira et al. (2016) stated that studies aiming at identifying AMF in E. edulis roots should consider longer seedling cultivation times. Following this recommendation, this study postponed evaluations over six months after inoculation, achieving effective mycorrhizal colonization percentages between 18 and 58%, depending on fungal isolates and P doses applied. Root colonization percentages of palm species reported in the literature range from 13 to 53% in Bactris gasipaes20,23, 4 to 33% in Desmoncus orthacanthos18, 27 to 86% in Phoenix dactylifera8, and 4 to 4.2% in Astrocaryum mexicanum16.
E. edulis plants inoculated with AMF accumulated higher N, P, K, Ca and Mg in shoots and roots 15.
Shoot biometry
Significant effects of fungal treatments and P doses applied were verified for the following biometric variables: shoot length (H), shoot diameter (SD), leaf area (LA) and dry shoot mass (Table 3).
Table 3: Tabla 3: Shoot length (H), shoot diameter, (SD), leaf area (LA) and dry shoot mass (DSM) of Juçara palm seedling as a function of arbuscular mycorrhizal fungi (AMF) and phosphorus content (P), 226 days after sowing. Altura del brote (H), diámetro del brote (SD), área foliar (LA) y biomasa seca del brote (DSM) de la plántula de palma Juçara en función de hongos micorrízicos arbusculares (HMA) y contenido de fósforo (P), 226 días después de la siembra.

Plants inoculated with R. clarus (IN1) and R. clarus + C. etunicatum mixed inoculum (IN3) increased shoot length (H) even in the absence of P (Table 3). At P dose of 50 mg dm-3, different inoculated and non-inoculated plants showed no significant difference in this variable. However, these treatments exhibited average lengths close to 30 cm, the commercial recommendation for the species according to Aguiar et al. (2002) (Table 3).
Average H was 25.66 cm, with variations between 21.33 cm (minimum for control treatment at 0 mg dm-3 P), and 27.70 cm (maximum for R. clarus treatment at 50 mg dm-3 P). In the absence of phosphate fertilization, fungal treatments containing R. clarus (IN1) and mixed inoculum did not differ from each other and showed the highest increases in H, 28% and 26%, respectively, when compared to control.
In the absence of P, fungal treatment with C. etunicatum (IN2) did not statistically differ from control, presenting the lowest average H, 21.33 cm and 22.48 cm, respectively. Under phosphate fertilization, fungal treatments did not differ from each other (Table 3, page 113).
The H values obtained in this work are higher than those obtained by Sgrott et al. (2012) and Moreira et al. (2016) when evaluating the effect of AMF on initial growth of E. edulis. At 160 days after inoculation, Sgrott et al. (2012) recorded average H of 15.52 cm for treatments inoculated with an AMF mixture (Acaulospora koskei, Scutellospora heterogama, Gigaspora albida, R. clarum), and 15.70 cm for control, with no significant differences between treatments. Moreira et al. (2016) recorded average H ranging between 16.15 cm and 18.25 cm at 180 days after inoculation, with the shortest stem recorded for control treatment and the longest for fungal treatment with AMF soil spores collected using the on-farm method, and finding no differences between treatments as well.
The absence of mycorrhizal effect on early stages of seedling growth can be explained by the large Juçara palm seeds. Venturi and Paulilo (1998) evaluated depletion of seed reserves and the effect of mineral nutrition on E. edulis seedlings finding that during initial growth stages, within the first five months after sowing, seedlings still depended on seed reserves.
Considering that the time required to reach these indexes is six to eight months 22, these results demonstrate the positive effects of inoculation with mycorrhizal fungi on growth of Juçara palm seedlings. Treatments with R. clarus (IN1) and mixed inoculum reached average values close to those recommended for seedling commercialization, in less time than expected.
Regarding stem diameter (SD), no significant difference was found among fungal treatments (AMFs) regardless P content (Table 3, page 113). However, when not inoculated, plants showed higher average SD under phosphate fertilization than in the absence of P. Seedling diameter is one of the most important quality standards 25. According to Duarte et al. (2015), seedlings with adequate SD show improved shoot growth balance. Larger diameters indicate better uptake and translocation of nutrients to the plant. According to Silva et al. (2015), although no precise standard for Juçara seedlings commercialization is defined, seedlings with a minimum SD of 5 mm are recommended, requiring six to eight months of nursery. Contrasting results show, on one side, no significant difference in SD of E. edulis seedlings 160 days after inoculation with AMF (Sgrott et al., 2012). On the other side, Moreira et al. (2016) did observe significant differences for this variable 180 days after inoculation with AMF, showing that, regardless of AMF used, the presence of mycorrhiza increased seedling diameter when compared to control treatment, without inoculation.
The LA of Juçara palm seedlings in the absence of P showed significant differences among treatments inoculated with R. clarus and mixed inoculum, obtaining the highest averages of 136.04 and 119.57 cm², with 80% and 58% increase, respectively (Table 3, page 113). Control and treatment with C. etunicatum (IN2) did not differ at 0 mg dm-3 P, showing the smallest values, 75.71 and 80.98 cm², respectively (Table 3, page 113).
In the presence of phosphate fertilization, treatments did not differ from each other. However, a significant increase in LA was observed for IN1 and IN3 treatments with C. etunicatum, (74% and 64%, respectively), at 0 mg dm-3 P (Table 3, page 113). These results hold mechanistic importance, since LA expresses the size of the photosynthetic apparatus, influencing carbon assimilation, plant growth and development 16. In Bactris gasipaes seedlings, LA of inoculated plants was significantly higher than that of non-inoculated plants, resulting in more developed plants and, therefore, earlier planting capacity and better performance expectations under field conditions 24. In contrast, assessing AMF contribution to initial growth and P absorption of Desmoncus orthacanthos Martius palm, Ramos-Zapata et al. (2006) observed that LA significantly responded to P treatments, but not to mycorrhizal treatment.
Dry shoot biomass (DSM) of Juçara seedlings inoculated with R. clarus and mixed inoculum, without P, was significantly higher than in control treatment, with 64% and 57% increments, respectively (Table 3, page 113). DSM of control plants and those inoculated with C. etunicatum (IN2) resulted in the lowest averages, 0.98 and 1.02 g, respectively, not differing significantly from each other at 0 mg dm-3 P (Table 3, page 113).
Under phosphate fertilization, average DSM of plants with R. clarus (IN1) (1.79 g) significantly differed from those with C. etunicatum (IN2) holding the lowest average (1.52 g), and not differing from the other treatments under the same P dose. The addition of 50 mg dm-3 of P increased seedling DSM (Table 3, page 113).
When producing E. edulis seedlings inoculated with mycorrhizal fungi, Sgrott et al. (2012) found no significant difference in DSM five months after inoculation under nursery conditions. However, after 24 months under field conditions, previously inoculated seedlings showed almost 50% increases in dry shoot biomass in relation to non-inoculated plants. Later, Moreira et al. (2016) evaluated initial growth of E. edulis seedlings inoculated with AMF and found that, regardless of the AMF species, inoculated seedlings showed higher dry shoot biomass than control treatment.
According to Rodrigues et al. (2018), greater plant growth after inoculation with mycorrhizal fungi reduces seedling production time and enhances initial growth after field planting. For heart of palm seedlings inoculated with AMF, Silva et al. (1998) recorded 140% increase in dry shoot biomass in relation to non-inoculated treatment.
Macronutrient content
Shoot nutrient content expresses the ability of AMF to increase soil nutrient absorption. In this work, N, P, K, Ca, Mg and S contents in dry shoot biomass of Juçara palm seedlings were influenced by P doses and fungal treatments (Table 4).
Table 4: Tabla 4: Macronutrient contents, (mg plant-1), of Juçara palm seedling as a function of arbuscular mycorrhizal fungi (AMF) and phosphorus content (P), 226 days after sowing. Contenido de macronutrientes, (mg planta-1), de plántula de palma Juçara en función de hongos micorrízicos arbusculares (HMA) y contenido de fósforo (P), 226 días después de la siembra.

In the absence of phosphate fertilization, macronutrient contents were statistically higher in Juçara seedlings inoculated with R. clarus (IN1) and mixed inoculum (IN3), than in control. Macronutrient content increments for treatments with R. clarus and mixed inoculum were: 61% and 56% for N; 249% and 225% for P; 61% and 69% for K; 71% and 71% for Ca; 93% and 87% for Mg; and 63% and 53% for S, respectively, when compared to control treatment (without inoculum at P dose of 0 mg dm-3) (Table 4).
N, K, Ca and S contents were not influenced by fungal treatments at 50 mg dm-3 P. Meanwhile, treatments with R. clarus (IN1) and mixed inoculum did not differ for P content but were statistically superior to control treatments without inoculation and with C. etunicatum (IN2) at 50 mg dm-3 P (Table 4, page 115).
At 50 mg dm-3 P, macronutrient contents of all treatments increased. However, in treatments with R. claruse and mixed inoculum, P application did not affect K and S contents (Table 4, page 115). This was also observed for Ca contents in treatments with mixed inoculum (Table 4, page 115). These results demonstrated that using mycorrhizal fungi was effective even in the absence of phosphate fertilization, evidencing economy of inputs for seedling production.
Moreira et al. (2016) recorded greater N, P, K, Ca and Mg accumulation in DSM of Juçara seedlings six months after inoculation with AMF, demonstrating the significant effect of this association for plant nutrition. Sgrott et al. (2012) also evidenced several benefits of mycorrhizal inoculation of E. edulis seedlings but did not evaluate shoot nutrient contents. Before, Lima et al. (2014) had evaluated growth of E. edulis seedlings in response to different P doses 12 months after phosphate application and concluded that P did not change plant nutrient content, finding the following nutrient absorption sequence in shoots: N> Ca> K> Mg> P> S, but not quantifying these nutrients in plants.
Studies involving associations between mycorrhizal fungi and plants have proven to be important. AMF improve plant nutritional status, promoted by a more efficient soil exploration, easier access to soil micropores by fungal hyphae and consequent higher nutrient absorption capacity. In addition, hyphae produce and exude organic compounds that facilitate phosphate solubilization, increasing nutrients availability 17. In this sense, Ait-El-Mokhtar et al. (2019) reported the importance of AMF in stabilizing K, P and Ca levels, and also Ca / Na, K / Na ratios in Phoenix dactylifera L. plants submitted to salt stress. This greater nutrient accumulation in plant tissues provides higher seedling quality and, consequently, greater chance of survival. In this respect, the use of inoculants with AMF results in more vigorous plants obtained by a low-cost production strategy. Moreira et al. (2016) reported that these benefits are even more significant for plants like E. edulis, since the species, now threatened with extinction, has difficulties germinating and stabilize in the field.
Conclusions
AMF, a biological agent of mutualistic associations with plants, constitutes an innovative approach to sustainable agriculture, contributing to increase plant survival rate, a key-factor for the successful reintroduction and conservation of E. edulis, as well as for its commercial exploitation.
Fungal species R. clarus and mixed inoculum (R. clarus + C. etunicatum) have high infectious potential, promote greater growth, and shoot dry matter accumulation of E. edulis plants.
Fungal species R. clarus and mixed inoculum (R. clarus + C. etunicatum) show associative preference with roots of E. edulis seedlings.
Inoculation with R. clarus and mixed inoculum (R. clarus + C. etunicatum), in the absence of P, contributes to greater N, P, K, Ca, Mg and S accumulation in E. edulis plants.














