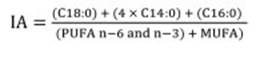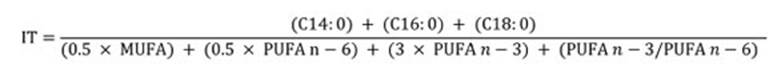Introduction
Fish is an important functional food for approximately three billion people 46. It provides high quality protein, essential minerals 31 and polyunsaturated long chain omega-3 fatty acids, especially eicosapentaenoic and docosahexaenoic acids, with high nutritive value 12. Therefore, fish quality (in terms of freshness and the mentioned nutritive value) has become a strategic priority for the industry, and its inclusion in the human diet is essential 30.
In Ecuador, fishery contributes 7% of total animal protein supply. Sixty five percent comes from capture fisheries, and the remaining 35% from aquaculture. This last activity is source of employment and foreign exchange, while contributing to rural settlement and food security 15. Also, rural aquaculture has become a key component of rural livelihoods in situations where increasing population, environmental degradation, or loss of access, limit catches from wild fisheries 14. The fish species mainly caught on the coastal and Ecuadorian Amazon areas are Cichlasoma festae, Andinoacara rivulatus, Prochilodus magdalenae, Brycon alburnus, Leporinus ecuadoriensis, Hoplias microlepis and Lebiasina bimaculata, among others 15. A. rivulatus (syn. Aequidens rivulatus) or Vieja Azul, whose meat is highly appreciated by local consumers, is a colorful fish from the cichlid family distributed in the coastal waters from the Tumbes River (Perú) to the Esmeraldas River (Ecuador). They are found in low flow environments, although some inhabit more fast-flowing rivers. Its diet is mainly composed of insects and crustaceans 17. Adult males and females may reach lengths of 30 cm.
In order to produce and preserve this native species, a conservation programme was developed by the Subsecretary of Aquaculture of the Ministry of Agriculture, Livestock, Aquaculture and Fisheries (MAGAP, according to its initials in Spanish). In addition to improving environmental conditions and reducing pollution, this program must support aquaculture development. According to Solórzano Armijos (2016), knowledge of wild populations is necessary for improving chemical, physical and nutritional traits, as well as sensory profiles in the farmed specimens 21,23,39. Although morphological comparisons between cultured and wild fishes from several species have already been approached by several authors 7,19,21,44, differences based on nutritional composition among cultured and wild A. rivulatus stocks, have not been studied yet. According to Mazon et al. (2018, 2020, 2021) and Gonzalez et al. (2017), productive yields of native species were influenced by genetic and environmental factors, like sex and rearing system (cultured vs. wild production). Consequently, this study aimed to evaluate the effect of rearing system and sex on the carcass and fillet traits, fatty acid composition and nutritional value of muscle tissue from A. rivulatus.
Materials and methods
Study area and experimental fish
The study was carried out in three areas of the Babahoyo River and a fish farm Center located in the Province Los Rios (Ecuador). The area has a tropical climate with average temperature of 25°C, annual rainfall of 2,400 mm and relative humidity of 82%. Water salinity did not exceed 0.1%, pH was 7.0-7.3 and temperature ranged between 19.7 and 24.7°C. Dissolved oxygen in the river and fish farm was 6.8 and 8.9 mg/l, respectively. Conductivity values were about 145 mS/cm.
Three hundred healthy adult specimens, male and female, of A. rivulatus were obtained at random from catches throughout year 2017. The sample included 300 adult specimens from Babahoyo River; 150 wild captured by local fishermen using nets, and 150 cultured. Farm rearing was carried out as follows: Three hundred and sixty initial specimens were cultured in net cages fixed in a pond bypass of the Babahoyo River. Eighteen net cages were fixed in a surface of 1 m2, submerged 1 m, and filled with 20 fish per m2, according to Rodriguez et al. (2017). In the farm, food was distributed three times/day adjusting consumption to 1.5% biomass. The diet was 32% crude protein, 7% fat, 5% crude fiber, 9% ash and 12% moisture. It was elaborated with cereal by-products, soybean meal, fish meal, corn protein concentrate, lecithin, vegetable oil, calcium carbonate, calcium phosphate, antioxidants and a premix of vitamins and minerals. From these 360 cultured fish, 150 were then sampled. Meanwhile, wild fish ate natural food based on insects and crustaceans. Males and females were morphologically differentiated.
All individuals were healthy adult fish, 2 to 3 years old, according to the number of scales, 13. In this research, no significant differences were found in number of scales according to rearing system and sex 7 ranging between 18.90 to 19.38 scales. Therefore, fish age was not considered a fixed factor.
After being caught, the specimens were kept in glass containers with 200 l dechlorinated tap water and continuous aeration. They were transported alive and introduced into two masonry tanks (capacity of 500 L, dissolved oxygen = 6.20 + 0.0 mg/L, temperature = 20.5 + 0.2°C and pH = 5.6 + 0.1). All fishes rested for 48 h before the experiment, with a fasting period of 24 h before stunning. For the experiment, water level in the tank was reduced by half; all fishes were quickly caught with a net and transferred to a plastic box (100 L), containing a mixture of 40 L of ice and 40 L of water (0.8°C), for stunning (about 20 min). Once death was certified, the fishes were identified, pH and biometric traits were performed and the specimens, in an undistorted condition, were stored in individual plastic bags at 0 + 2°C in ice, until further analysis and processing.
pH determination
Muscle pH was determined in duplicate after death (pH0), at 2 hours (pH2) and 12 hours (pH12) post mortem by inserting a pH electrode (portable meat pHmetre, HI99163, Hanna Instruments Ltd, UK) approximately 1 cm into the fillet’s right dorsal-cranial portion. The instrument was frequently calibrated using pH 4.01 and pH 7.00 buffers, and the electrode regularly cleaned to obtain consistent results.
Biometric and yield parameters
After rigor mortis, approximately 24 h post mortem, the fishes were weighed (bodyweight), measured (total and standard length) and dissected with a scalpel and scissors. Fins, scales, head, entrails, bones and fillet were removed and weighed. Head, guts and skin + fins yield was calculated according to Rutten et al. (2004). In addition, slaughter yield, dress-out, fillet yield and condition factor (K) were estimated by the following equations:
Flesh quality
After 45 minutes of filleting, surface colour of the internal side of right fillets was recorded at three positions using a portable colourimeter (Lutron RGB-1002 Chroma Meter) equipped with light source C and a 2° observed angle, calibrated to a white standard. Colour variables were: L* (lightness, +L* = white, -L* = black), a* (red-green chromaticity, +a* = red, -a* = green) and b* (yellow-blue chromaticity, +b* = yellow, -b* = blue) as recommended by CIE (1976). For each fillet, three measurements (along the fillet) were done. Water holding capacity was determined according to Grau & Hamm (1953), in two ways: drip loss and cooking loss. To determine drip loss, two pieces of 5 mm × 10 mm × 20 mm fresh muscle, were cut. These cubes were carefully suspended with a pin on the inside of a bottle cap, not touching either side of the bottle, and stored for 24 h at 2 +°C. The amount of drip measured between 24 h and 48 h post mortem was expressed as percentage of the initial weight: 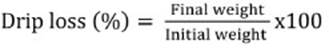 . To evaluate cooking loss, the samples (approximately 30 g) were trimmed of external fat, weighed, placed in a polyethylene bag and immersed in a water bath (JP Selecta, Barcelona, Spain) at 80°C until internal temperature achieved 70°C. Temperature was repeatedly monitored by a Type flexible high-temperature thermocouple (Hanna, Instruments, EE.UU) inserted into the geometric centre of each piece. Once the samples were cooled at room temperature (approximately 15°C) for 40 min and gently dried on filter paper, they were re-weighed. Cooking loss percentage was calculated as follows:
. To evaluate cooking loss, the samples (approximately 30 g) were trimmed of external fat, weighed, placed in a polyethylene bag and immersed in a water bath (JP Selecta, Barcelona, Spain) at 80°C until internal temperature achieved 70°C. Temperature was repeatedly monitored by a Type flexible high-temperature thermocouple (Hanna, Instruments, EE.UU) inserted into the geometric centre of each piece. Once the samples were cooled at room temperature (approximately 15°C) for 40 min and gently dried on filter paper, they were re-weighed. Cooking loss percentage was calculated as follows:
Proximate composition and fatty acid profile
For proximate composition analysis, A. rivulatus fillets were homogenized using a 20.000 rpm grinder. Crude protein and fat content were measured by the block digestion method (UNE 55-020), ash was obtained at 550°C for 24 h (ISO R-936), and wet percentage was determined by drying at 103°C for 24 h (ISO R-1442) according to AOAC (2000). Fat percentage was measured according to the Soxhlet method (ISO R-1443) using a Foss Tecator AB Soxtec 2050. Analyses were determined in duplicate and expressed as mg/100g of raw meat.
Skinned and deboned muscles from individual fish, were blended into homogeneous flesh for total lipid extraction with chloroform/methanol (2:1 v/v) containing 0.01% of Butylated hydroxytoluene (BHT) as antioxidant 16. The organic solvent was evaporated under nitrogen stream and lipid content was determined gravimetrically. Aliquots of extracted lipids were converted to fatty acid methyl esters (FAME) according to Chistie (1993). FAME were separated and identified on GC Perkin Elmer Clarus 500 gas chromatograph with a flame ionization detector (FID) equipped with a TR-FAME capillary column (30 m x 0.25 mm i.d., 0.25 μm film thickness, Shinwa Inc.), using helium as carrier gas at a flow rate of 0.5 ml/min. Both injector and detector were maintained at 250 and 260°C, respectively. Oven temperature was programmed at 100°C, followed by increasing steps of 2°C/min until 220°C, with a final hold time of 20 min. Individual fatty acids were identified by comparing their retention times with those of a standard fatty acid mix Sulpeco 37 (Sigma Chemical Co. Ltd., Poole, UK). Nonadecanoic acid methyl ester (19:0 ME) was used as internal standard. Individual fatty acids were expressed as percentage of total identified fatty acids, and as mg/g muscle raw tissue. They were grouped as follows: saturated fatty acid (SFA), monounsaturated (MUFA), polyunsaturated fatty acid (PUFA), n-6 and n-3. The PUFA/SFA, DHA/EPA, Σ n-6/Σ n-3 ratios, atherogenicity (IA) and thrombogenicity (IT) indices were also calculated. IA indicates the relationship between the sum of the main saturated fatty acids and that of the main classes of unsaturated, the former being considered pro-atherogenic (favouring the adhesion of lipids to cells of the immunological and circulatory system), and the latter anti-atherogenic (inhibiting plaque aggregation and diminishing levels of esterified fatty acid, cholesterol, and phospholipids, preventing micro and macro coronary diseases). Finally, IT shows clotting tendency in blood vessels 42. IA and IT indices were calculated by using the Ulbricht and Southgate (1991) equations as follows:
Statistical analysis
A total of 300 fish flesh samples were analysed according to different parameters. Normality and homoscedasticity were checked with Kolmogorov-Smirnoff and Levene tests, respectively. Then, general linear models (GLM) with post hoc Tukey test determined effects of rearing system (wild and cultured) and sex (male and female) on carcass and fillet traits, proximate composition, fatty acid composition and trace mineral content. Differences were considered statistically significant for p < 0.05. All statistical analyses were done using Statistica 12.0 for Windows.
Results and discussion
Biometric and yield parameters
Live weight was significantly higher in the wild population (Table 1), which seems to indicate that artificial feeding should be improved. However, the cultivated population had a greater total length, probably a morphological adaptation to this aquatic habitat.
Table 1: Tabla 1: Biometric measurements and yield parameters (mean value + SE) by rearing system and sex of A. rivulatus. Medidas biométricas y rendimientos (media ± ES) en cada sistema y sexo de A. rivulatus.
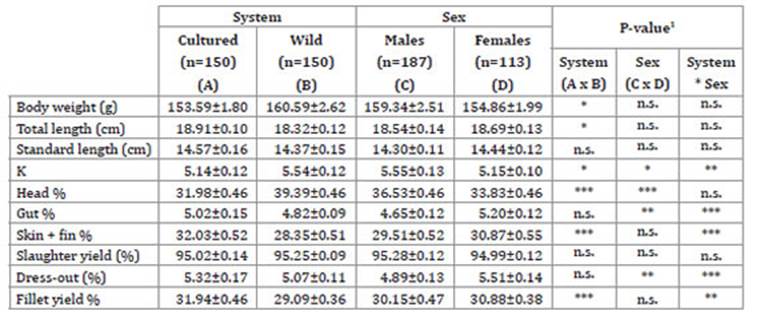
1 * p < 0.05; ** p < 0.01; *** p < 0.001; n.s.: not significant.
1 * p < 0,05; ** p < 0,01; *** p < 0,001; n.s.: no significativo.
These results partially agree with Vreven et al. (1998) who also indicated that confinement of domesticated fish affects their growth rate, but contrasting with our results as they observed no body elongation, along with a higher K value. Sex had a non-significant influence on both variables, attributable to the fact that catch size is conditioned by consumer demand. Rearing system and sex had a significant effect on the K factor, in agreement with González et al. (2016) in Cichlasoma festae. All wild fish and cultivated males presented higher values than all those cultivated and females. The K values obtained in this study, indicators of the status of A. rivulatus aquatic ecosystems, were higher than that recorded by Anene (2005) in four cichlid fish (4.9), and those registered for González et al. (2016) in Cichlasoma festae (3.01 and 3.62, for cultured and wild specimens, respectively) in similar habitats to those of the present study. This evidences a greater feeding capacity than the species previously mentioned.
Rearing system significantly affected head (higher in wild specimens), skin + fin and fillet yield percentages (higher in cultivated specimens), in accordance with Gonzalez et al. (2017). Meanwhile, sex significantly affected head and slaughter + end (higher in males), and gut and dress-out percentage (higher in females). Head percentage was significantly higher in wild fish, while skin + fins and fillet percentages were higher in cultured specimens. These results were similar to those found by Gonzalez et al. (2016) for Cichlasoma festae in Ecuador. Differences between systems could be attributed to fish high phenotypic plasticity, greater than any other vertebrates 6.
Flesh quality and proximate composition
Flesh quality traits of A. rivulatus are shown in Table 2.
Table 2: Table 2: Flesh quality traits, water holding capacity (WHC), and proximate composition (g/100 g wet weight) (mean value + SE) by rearing system and sex of A. rivulatus. Características de calidad de la carne y capacidad de retención de agua (WHC) y composición proximal (g/100 g peso húmedo) (media ± ES) en cada sistema y sexo de A. rivulatus.
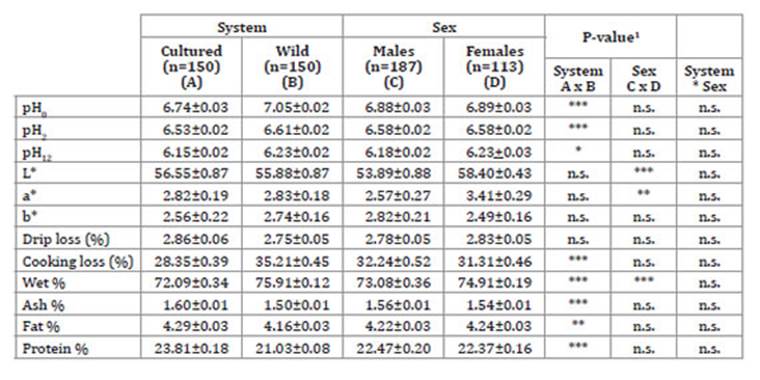
pH0 = pH at slaughter; pH2 = pH at 2 hours post mortem; pH12 = pH at 12 hours post mortem; L*, a* and b* = instrumental parameters color (CIE L*. a*. b*) 1 * p < 0.05; ** p < 0.01; *** p < 0.001; n.s.: not significant.
pH0 = pH al sacrificio; pH2 = pH a las 2 horas post mortem; pH12 = pH a las 12 horas post mortem; L*, a* and b* = parámetros del color (CIE L*. a*. b*) 1 * p < 0,05; ** p < 0,01; *** p < 0,001; n.s.: no significativo.
The pH0, suitable index of meat quality 25, constitutes the most important factor influencing meat texture. Minor changes in pH dramatically modify both its physical and sensory properties.
In the first 12 hours post mortem, pH values were lower in wild specimens than in cultivated ones (0.83 vs. 0.59), in accordance with Robb et al. (2000) and Roth et al. (2009) who reported that muscle pH displayed a rapid decline during the aforementioned period. However, this drop was linear and progressive, in disagreement with González et al. (2017), who had indicated a harsh drop during the first 2 hours post mortem. Even though pH values were adjusted by the temperature sensor of the pHmetre used, temperature may have generated differences in pH. High values were associated with small and stressed animals 8,28 and influenced by rearing system, with higher values in wild specimens, to which capture procedure could contribute.
Drip loss resulted similar to the obtained by González et al. (2017), although lower than those reported by Intarak et al. (2015) in Panga fish (Pangasius bocourti Sauvage), who obtained values from 4.88% to 2.88%, with significant decreases as live weight increased. None of the studied factors, rearing system or sex, had significant influence on drip loss. Cooking loss was similar to the recorded by Gonzalez et al. (2017) in Cichlasoma festae. These authors did not find system-attributable differences, while in our study wild specimens flesh showed significantly higher values, possibly due to higher wet content.
Fish composition is affected by several factors such as size, temperature, salinity, rearing system and feeding, among others 18. According to Chandrashetkar and Desthale (1993), normal variations between fish constituents are: 66-81% wet, 16-21% protein, 0.2-25% fat and 1.2-1.5% ash. Proximate analysis (Table 2, page 237) resulted similar to that reported by Mashaii et al. (2012) for ash (1.38-1.59%), wet percentage (75.2-76.9%) and total fat (2.48-4.88%), while resulted higher than protein content (17.4-17.9%) in cultured Oncorhynchus mykiss. Concerning crude protein content, our results were also higher than those obtained for Cyprinus carpio (16% wet weight) 14 and for Cichlasoma festae (17.33% wet weight) 20 but similar to the 18.4-20.8% reported for the Cichlidae family 36. Gonzalez-Artola (2004) classified fish according to fat content, into lean fish (fat less than 2%), low-fat fish (fat 2-4%), medium fat fish (fat 4-8%) and high-fat fish (fat more 8%). Thus, based on this classification, A. rivulatus could be considered a medium fat fish. In agreement with previous studies 1,5,20,31, rearing system significantly influenced every analyzed variable. Cultured specimens possessed higher ash, fat and protein, and lower wet percentage than wild ones. Female fish flesh had higher wet percentage than males. Differences between culture and wild specimens could probably be due to a high dietary protein level in cultured fish feed.
Fatty acid profile
Fish body composition suffers changes in response to diet and environmental conditions. Differences in fatty acid composition between rearing systems could be attributable to differences between cultured and wild specimens. Significant differences in fatty acid content were found between cultured and wild fish, except for ratio n-3/n-6, which was similar between both systems (Table 3, page 239).
Table 3: Table 3: Fatty acid composition (mean value + SE) according to rearing system and sex of A. rivulatus. Composición de ácidos grasos (media ± ES) en cada sistema y sexo de A. rivulatus.
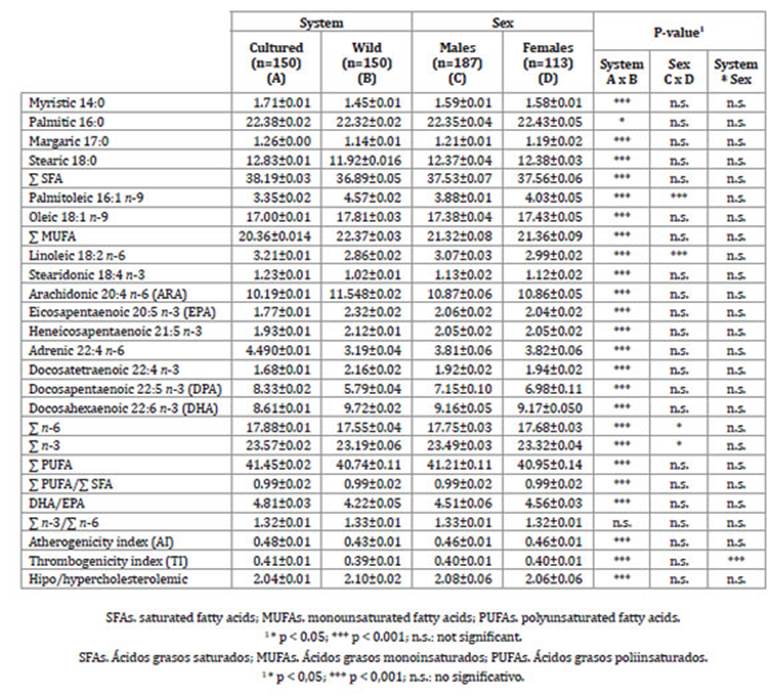
However, sex had little influence on fatty acids profile. SFA and PUFA percentages were higher in cultured fish, whereas MUFA content, was lower, probably given to the low content of oleic and palmitoleic acids in cultured fish feed. Assimilation patterns of dietary fatty acids in fish muscle reflect the content of dietary lipid sources 1. The major fatty acids identified in both fish were palmitic, oleic, stearic, arachidonic, docosahexaenoic and docosapentaenoic acids.
Palmitic acid was the primary saturated fatty acid, contributing, approximately, with 58.6% and 60.8% of total SFA content for cultured and wild fish, respectively, lower than those obtained by Alasalvar et al. (2002). Meat from cultured specimens had a higher SFA content than that of wild ones (40.18% vs. 38.33%), in accordance with Łuczynsk et al. (2014). Thus, the remaining fatty acids found in both fish (about 56%) were mono and polyunsaturated fatty acids.
Oleic acid was identified as the primary MUFA in both rearing systems, significantly higher in wild fish, in accordance with Alasalvar et al. (2010). This fatty acid has an exogenous origin, probably owed to the diet 27.
Regarding PUFA content, significantly higher levels were found for cultured fish. According to Martínez et al. (2010) and Busetto et al. (2008), n-6 fatty acid content was significantly higher in cultured fish. Arachidonic acid was the dominant fatty acid in both fish, significantly higher in wild fish and greater than that obtained by Łuczynsk et al. (2014), Jabeen et al. (2011) and Martínez et al. (2010). This acid is a prostaglandin and thromboxane precursor 37, probably facilitating blood clotting by attaching to endothelial cells during wound healing.
In the n-3 fatty acids family, docosahexaenoic and docosapentaenoic acids were the most abundant, achieving greater values than for C. festae in similar climatic conditions 20. However, eicosapentaenoic acid values were lower than those obtained by Gonzalez et al. (2017) for C. festae in Ecuador. Given that DHA and EPA are key components for human healthy diets 29, suitable choices of dietary lipids in cultured fish would allow improving fatty acids profile, especially in n-3 PUFAs.
In our study, no differences between rearing systems were found in n-3/n-6 ratio (1.32 for all data). These values were higher than those obtained by González et al. (2017) in C. festae and Hoseini et al. (2013), in farmed Hypophthalmichthys nobilis and Ctenopharyngodon idella. Simopoulos (2008) suggested that n-3⁄n-6 ratio should be kept between 1:1 and 1:4.
Nutritional quality was evaluated through atherogenicity (IA) and thrombogenicity (IT) indices determining the potential impact on human health. Mean values of IA and IT indices were 0.48 and 0.41 for cultured fish and 0.43 and 0.39 for wild specimens, lower than the reported by Šimat et al. (2015). However, in contrast with Šimat et al. (2015), in cultured fish, IA and IT were significantly higher.
Conclusions
Rearing system and sex significantly influence most of the analyzed characteristics of carcass and flesh of A. rivulatus. High fillet yield and its proximate composition categorize A. rivulatus as a suitable food. The results obtained from this study indicate that proximate composition of fillet of cultured A. rivulatus is more adequate than those of wild A. rivulatus, since cultured fish contains higher n-3 and n-6 PUFA percentages and adequate n-3/n-6 ratio.