Introduction
Palms are among the most cultivated plants worldwide. The species domestication, linked to the development of agriculture, represents a significant step in human development 3,22.
The species Phoenix roebelenii O’Brien is indigenous to the Southeast of Asia. In Brazil, known as dwarf date palm or phoenix palm, is widely used in landscape design projects, parks, gardens, streets and interior design. It is mainly appreciated for its height, exuberant leaves and edaphoclimatic adaptability 9,20,21.
Following seed dispersal, germination is one of the most important physiological mechanisms for perpetuation of the species 6, defining the existence of a community in a certain habitat. Nevertheless, for germination success, several specific environmental factors, such as water, light and suitable temperature must range within certain values 7.
Eric Roberts, studying seeds storage, proposed a definition for recalcitrant seeds in the 70s, 1. Seeds showing tolerance to desiccation and storage at negative temperatures were called orthodox, while seeds that lost their viability when subjected to these conditions, were called recalcitrant 1. For orthodox seeds, during the latest stages of maturation, when desiccation occurs, metabolism becomes quiescent, and orthodox seeds gain desiccation tolerance, allowing seed storage without viability loss 4. However, despite the low metabolic activity after seed desiccation, seeds do deteriorate over time, becoming evident and quantifiable, after rehydration 16.
In this sense, desiccation tolerance (TD) can be defined as seed capability to maintain viability after loosing cell water content without showing irreversible damages 14,22. Additionally, seed critical water content can be understood as the minimal water content ensuring survival, that is, without irreversible damage. This value is approximately, 0.07 g H2O g dry weight-1 for orthodox seeds and 0.2 g H2O g dry weight-1 for recalcitrant seeds 24. Thus, TD is an evolutionary characteristic allowing growth and reproduction even after severe dehydration, sometimes above 90% 12.
Variations in seed responses to storage suggest the existence of complex mechanisms. Their understanding inspire new technologies ensuring seed availability along time 24.
The present research is motivated by the interest in P. roebelenii as an ornamental palm and the need to preserve the species variability. Our objective was to analyze seed physiological quality after desiccation and storage.
Material and methods
Study area, seed harvesting and processing
Fruits of P. roebelenii were manually harvested from plants grown in private gardens in Campos dos Goytacazes, Rio de Janeiro - Brazil. Fruits were immersed in water for 72 hours. Nextly, all seeds were manually extracted by friction in a steel sieve, removing all residues by rinsing the seeds in running water. Seed surface was disinfested with 2% sodium hypochlorite for 5 minutes. After rinsing, excess water was removed with toilet paper and seeds were left to air dry in the shade, for 24 hours.
Physiological classification after desiccation and storage
The physiological classification of P. roebelenii store seeds was based on Hong and Ellis (1996), adjusting the drying temperature in the convection oven to 35±2°C. Firstly, initial seed water content was determined as described in Brasil, Ministério da Agricultura, Pecuária e Abastecimento (2009). Afterwards, each step of the protocol was carried out with eight replications, each with 50 seeds, corresponding to the different seed lots. Desiccation time was estimated using the equation for viability evaluation 11:
where:
MR = sample mass reaching the desired moisture content
Ti = initial water content of the seed lot
Td = desired water content
Mi = initial mass of the seed sample 11.
At 12% water content, immediately after drying, 400 seeds separated in 16 fractions of 25 seeds, were used for seed viability evaluation. Emergence tests were conducted according to the methodology described below. The remaining seed lots, 200 seeds, remained in the desiccation chamber (convection oven) until reaching 5.03% seed water content. Then they were split in two sets, one for a new emergence test, while the other with 5.03% water content, was kept in a glass jar at -20°C, for 90 days. After this storage period, another germination test evaluated their physiological quality.
Desiccation and treatments
After mesocarp extraction, 33.92% seed initial water content was determined as described in Rules for Seed Analysis 5. The other treatments (33; 30; 25; 23; 20; 18; 16; 12; 10 and 7% water content) were obtained by monitoring seed water loss after adjusting oven temperature to 35±2°C, adapting the methodology proposed 11.
In the drying process, groups of 25 seeds were laid in gerbox boxes, all with initial known masses. The drying process was monitord by periodical weighting at one-hour intervals during the first 12 hours; and at six-hour intervals until the final desired seed water contents. For this purpose, the Hong and Ellis equation was used 11. Once the desired water contents were reached, the sample was homogenized, separated in 16 fractions of 25 seeds and sealed in hermetical glass flasks in a refrigerator at 7°C, temporarily, until all treatments were processed, that is, at 72 hours after starting seed desiccation.
Physiological evaluation of seed quality
Firstly, seed water content was determined by weighing (10-4 g precision) 30 replications with 30 seeds each. Then, the seeds were oven-dried at 105 ± 3°C, for 24 hours 5. The results were expressed in average percentage of humidity. Afterwards, the mass of a thousand seeds was determined as in Brasil, Ministério da Agricultura, Pecuária e Abastecimento (2009).
Germination tests were carried out with 16 subsamples, 25 seeds each. Seeds were sown on two layers of germitest paper and covered with a third layer. All paper layers had been previously imbibed with distilled water, 2.5 times their weights. Germination rolls were kept in a gemination chamber (BOD type), with 16/8 hours photoperiod, at 30°C.
Daily counting of normal seedlings 5, was then expressed as germination percentage. Seeds were considered germinated at primary root protrusion ≥ 2.0 mm. Concomitantly, the first germination was registered as the number of seeds germinated at 20 days after sowing. Germination speed index (GSI) was calculated as proposed by Maguire (1962). Number of germinated seeds was daily monitored until day 45 after sowing, after which it remained constant.
Seedling morphological evaluations
At 65 days, the following evaluations were made: i) length of the longest root (cm), from collar to tip of the longest root; ii) root/shoot length (cm), area (cm2), volume (cm3) and diameter (mm) were obtained using images and the WinRhizo Pro 2007a software, coupled to a scanner. iii) eophyll length - measured with a digital calliper, between the base and the apex; iv) collar diameter, measured with a digital calliper at seedling base; v) total fresh and dry seedling weights. Dry weight was obtained by conditioning each separate seedlings in Kraft paper bags kept in a convection oven at 65°C, until constant weight. Fresh and dry weights were determined with a 10-3 g precision scale.
Statistical analyses
The experimental design consisted of completely randomized blocks with 10 treatments (water content %) and eight replications of 25 seeds. Data were subjected to analysis of variance and means were compared by Tukey test at 5% probability, prior normality and homoscedasticity verification. Data analyses were done with R Core Team software.
Results and discussion
P. roebelenii seeds had an initial water content of 33.9% (Figure 1) and were desiccated for 72 h suffering an abrupt drop of water content in the first 15 hours, reaching, approximately, 10.0%.

Figura 1: Contenido de agua (%) de semillas de Phoenix roebelenii sometidas a desecación en un horno de convección a 35°C. Significativo al 5% (p < 0,05) de probabilidad.
Later, at 36 hours, a relative stabilization in seed water loss was achieved with about 7.0%, finally dropping to 5.03% at 72 hours (Figure 1).
Data show that P. roebelenii seeds preserved certain germination stability after reduced water content and storage (Table 1).
Table 1: Mean water content (%) and germination of Phoenix roebelenii seeds subjected to desiccation and storage.

At 33.92% water content, germination was 87.5%, reaching the highest percentage (92,5%) at 10.0% water content. Even after desiccation and hermetic storage for 90 days at -20°C, P. roebelenii seeds showed 88.0% germination (Table 1), indicating high desiccation tolerance and adequate storage at low temperatures. Thus, these seeds can be classified as orthodox 11. However, studying desiccation tolerance (TD) and viability during seed storage of the S. schizophylla palm, decreasing seed moisture from 21.3 to 11.6% caused more than 50% drop in germination percentage 3. Furthermore, no germination occurred below 11.6%, allowing S. schizophylla seeds to be classified as recalcitrant 3. In brief, different palm species show different behaviors in relation to seed recalcitrance, requiring different methodologies for storage and propagation. Studying seed desiccation sensitivity in Oenocarpus bacaba palm, reported that germination is significantly influenced by water content 2. At 41.7% moisture, O. bacaba seeds presented 70% germination. However, at 26.7%, they showed 100% mortality. These authors also reported that, in more developed seedlings, plant organogenesis, such as eophyll and cataphyll, is impaired 2. In this context, seed high water content during storage can favor deterioration processes by increasing respiration, followed by membrane degradation, microorganisms proliferation, and reduced germination and vigor 1,4. On the other hand, investigating desiccation tolerance of Butia odorata seeds, Fior et al. (2019) concluded an orthodox behavior, reaching a viability index of 90% at 6.14% moisture. Furthermore, Nascimento et al. (2010) observed no physiological effects on Euterpe oleraceae, seeds, cultivar BRS Pará, for up to 37.4% seed desiccation. However, water contents below 37.4% progressively favored deterioration, achieving null seed viability at 15.1%. The authors reported seed conservation of 270 days after storage at 37.4% water content and 20°C.
Desiccation tolerance can be defined as seed capability to maintain viability after losing cell water content without presenting irreversible damages 14,22. Table 1 indicates that P. roebelenii seeds did not suffer significant viability or vigor loss. Nevertheless, ongoing research has modified seed classification as recalcitrant, intermediary, or orthodox. Given the varied responses to desiccation and temperature tolerance, along with viability maintenance, different degrees of recalcitrance have been proposed, considering longevity during storage, dormancy and vigor 1.
Furthermore, many authors have acknowledged the need for new technologies allowing seed classification according to desiccation tolerance. Researchers state that besides TD and longevity, any characteristics that might distinguish different degrees of recalcitrance should be considered for seed classification 1. For instance, habitat, water content at 50% seed viability, dormancy, germination percentage immediately after dispersion, and maturation degree, among others. Previous reports on other Phoenix palm species considered Phoenix dactylifera seed as orthodox and Phoenix canariensis, Phoenix rupicola, Phoenix sylvestris and Phoenix reclinata as probably non-orthodox or intermediate 18,19.
Table 2 contains the descriptive statistics: mean and extreme values, and coefficient of variation for first germination count (FGC), root length (RL), root superficial area (SARS), root diameter (DR), root volume (VRS), seedling fresh weight (TFW) seedling dry weight (TDW).
Table 2: Mean and extreme values, and coefficient of variation (CV) for first germination count (FGC), root length (RL), root superficial area (SARS), root diameter (DR), root volume (VR), total seedling fresh weight (TFW) and seedling total dry weight (TDW) of Phoenix roebelenii.

Legend: ns = non-significant. * Significant at 5% (p<0.05) probability.
Leyenda: ns = no significativo. * Significativo al 5% (p<0,05) de probabilidad.
None of them were significantly affected by the treatments at the significance level of 5% probability. Only the FGC and TWD variables did not meet the normal distribution assumption.
The FGC coefficient of variation (CV) was extremely high (47.49%). This variable, along with TDW did not show normal distribution (Table 2). The RL had a low CV (8.99%). SARS, DR and VR showed intermediate values, 1874 cm2, 0.53 cm and 0.23 cm3; respectively (Table 2). FDW showed normal distribution (Table 2).
Germination percentage (PG) and gemination speed index (GSI) significantly increased with decreasing seed water content. When seed water contents ranged between 5 and 12%, the highest percentages of germination were observed (Figure 2A, page 31).
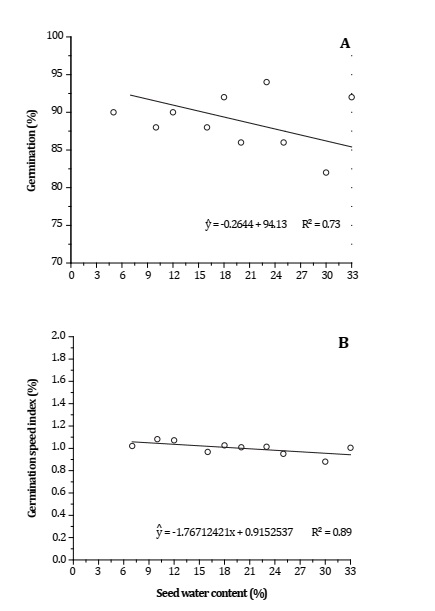
Significant at 5% (p < 0.05) probability. Significativo al 5% (p < 0,05) de probabilidad
Figura 2: Porcentaje de germinación de semillas (A) e índice de velocidad de germinación (B) de semillas de Phoenix roebelenii en función del contenido de agua de las semillas.
The highest GSI and IVG (1.14 and 1.15) were observed when seed water contents were 10 and 12%, respectively (Figure 2B, page 31). These indices represent key information for establishing normal and vigorous seedlings, indicating seed physiological quality 15.
The results indicate that P. roebelinii seeds are orthodox. This considers critical water content as the lowest water content allowing survival or not causing irreversible damage. This value is approximately 0.07 g H2O g dry weight-1 for orthodox seeds and, approximately, 0.2 g H2O g dry weight-1 for recalcitrant seeds 24. In P. roebelenii seeds, 98% PG was reported when seed water content was 30% 18, while at 8%, germination dropped to 90%. However, Caryota urens L. seeds with an initial moisture content of 34%, presented PG of 95%, whereas total loss of germination was observed at the lowest level (water content of only 29%).
According to Beltrame et al. (2018), considering different categories of orthodox, intermediate and recalcitrant seeds, with different levels of TD, requiring specific conditions to maintain physiological quality and ensure viability over time, turns indispensable.
Collar diameter (CD) of P. roebelenii seedlings at 65 days after sowing, decreased as a function of desiccation. Significant differences were observed in treatments in which seed water contents were under 18%, resulting in CD values between 1.72 and 1.85 cm, approximately. However, treatments with seed water contents above 18% (18-33%), led to non-significant CD values, ranging from 1.87 to 1.93 cm, (Figure 3A, page 32).

Significant at 5% (p < 0.05) probability. Significativo al 5% (p < 0,05) de probabilidad.
Figura 3: Diámetro del collar (A) y longitud del mesófilo (B) de plántulas de Phoenix roebelenii en función del contenido de agua de la semilla.
On the other hand, eophyll lengths of P. roebelenii seedlings were longer as seed water contents were reduced (Figure 3B), and significantly higher fore seed water contents under 18%, varying from 7.87 to 8.15 cm (Figure 3B).
The maximum storage time reached without seed viability loss is defined as longevity. In this context, seed longevity can be determined by the rate at which germination potential is lost over time, culminating with seed deterioration, that is seed capability of originating well formed and developed seedlings 17,23. Therefore, it can be inferred that P. roebelenii seeds could maintain their viability, originating normal seedlings.
Studies involving physiological, genetic and biochemical mechanisms for abiotic stress tolerance (salinity and temperature) in P. dactylifera plants, guide cultivar development 10. According to Walters (2015), variations in seed response to storage and desiccation imply the existence of complex mechanisms and, bring about new technologies leading to understand viability regulation, ensuring seed availability.
Conclusions
P. roebelenii seeds are orthodox.
Reduced seed water content increased the percentage of germination, the germination speed index, and eophyll length of P. roebelenii seedlings.
Reduced seed water content did not affect first germination count, root length, root area, root diameter, root volume, and total fresh and dry weight of P. roebelenii seedlings.
Decreased seed water content resulted in reduced collar diameter of P. roebelenii seedlings.















