Introduction
Spraying is widely used in agriculture for plant protection. For pesticide application, a liquid and an active diluted ingredient (phytosanitary product) form a syrup applied towards a target (weed, pest or pathogen) as a homogeneous cloud. The liquid usually used is water, the universal solvent 37. Water quality is essential, as it generally represents around ninety-five per cent of the spray solution. Therefore, chemical and physical elements in water can affect spray quality 18. In this sense, suspended materials such as silt, clay, and organic matter provide a “cloudy” aspect 23.
Soil sorption coefficient (Kd) and soil organic carbon-water partition coefficient (Koc) constitute pesticide-related coefficients that reflect pesticide force to be adsorbed to different particles. Pesticides with high Kd or Koc values bind strongly to sediments and organic matter in water, reducing active ingredients in the solution and reducing effectiveness 12. In this sense, several elements can influence application efficiency and effectiveness, such as Calcium, Magnesium, and Iron, free ions in the spray syrup 38. Accordingly, water hardness is a measure of total Calcium and Magnesium in water, reducing. pesticide effectiveness 13. Considering ion content, the pH also influences active ingredient availability in spray syrup. Acid/base ionization constants (pKa or pKb) represent ionization trends in a given pH range, determining syrup ingredient concentrations in the ionizable form directly affecting product effectiveness 12. Waters from rural regions may present dissolved ions or salts. River and weir waters have sediments such as clay and organic matter that can clog nozzles and filters, reducing spray components life 30. Thus, water choice is important for efficient spraying, especially considering water obtained from open reservoirs, such as rivers and weirs 20.
Another factor involved in application quality is product association in the sprayer tank. Products used in Integrated Pest Management or plant protection programs do not have a wide enough spectrum of action to effectively control all crop pests, making it necessary to mix products 21. Thus, farmers adopting this practice, face certain difficulties like product dissolving, foaming, precipitation, and flocculation, among other physical and chemical incompatibilities 15,16.
Given these facts, this work evaluated spraying syrup physical-chemical properties when formulated with different water qualities and tank-mixing multiple pesticides.
Material and methods
The experiments were conducted in laboratory and field. Field evaluations were located in the southern State of Mato Grosso do Sul, 22°27’04” latitude S and 55°01’27” longitude W, in Caarapã district, located in the Laguna Carapã city, Brazil. The climate is Monsoon (Am), according to Köppen’s classification 3, with dry winters, average annual rainfall of 1500 mm, and average temperature of 22°C (Figure 1).
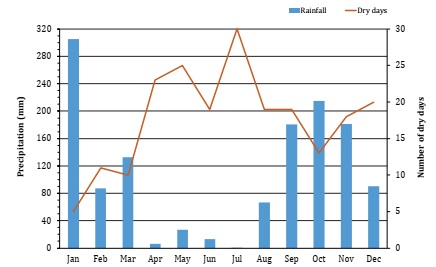
Adapted from National Institute of Meteorology (INMET, https://portal.inmet.gov.br/). Adaptado del Instituto Nacional de Meteorología (INMET, https://portal.inmet.gov.br/).
Figura 1: Lluvias mensuales y días secos para Laguna Carapã, Brasil, 2018.
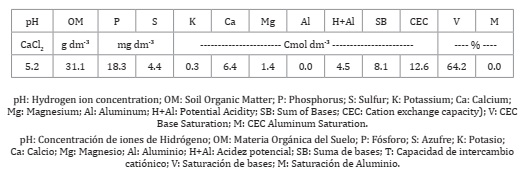
The soil is an Oxisol, with the chemical properties of a Clay soil (0 to 20 cm) under no-tillage (Table 1).
Table 1: Chemical soil analysis of the Santa Lúcia farm (0 to 20 cm) in Laguna Carapã, Brazil, 2018.
Soil sampling was carried out at Santa Lúcia farm, located in Laguna Carapã, Brazil.
The experimental design was completely randomized, in a 4x5 factorial scheme, with four replications. The treatments combined four water qualities (well, river, weir, and deionized water) and five pesticide tank-mixings (3 associations, one with herbicide and an untreated control). All treatments are described in Table 2 (page 135).
Table 2: Treatments combining water quality and pesticide tank-mixing.

* Tank-mixing 1: herbicide + insecticide; tank-mixing 2: herbicide + fungicide; tank-mixing 3: herbicide + insecticide + fungicide.
* Mezcla de tanque1: herbicida + insecticida; mezcla 2: herbicida + fungicida; mezcla 3: herbicida + insecticida + fungicida.
Table 3 describes phytosanitary products used in spray syrups, formulation, dosage, and composition of active ingredients.
Table3: Characterization of phytosanitary products.
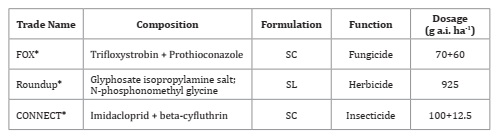
* AUREO: Soy methyl ester (Adjuvant), 0.25%vv; SL: soluble concentrate; SC: flowable suspension concentrate.
* AUREO: Éster de soja metilado (coadyuvante), 0.25%vv; SL: concentrado soluble; SC: suspensión concentrada.
These pesticides were selected for being the most used in the region.
The experiment was conducted in three sampling periods within a year. The first sampling corresponded to the off-season corn crop analyzed in April 2018. The second sampling was done during the pre-planting soybean weed control, while laboratory analyses were performed in September 2018. The third sampling corresponded to the soybean crop season, and the analyses were conducted in November 2018. All samples were analyzed on sampling date.
Three collecting sites were defined for water sampling, considering the spots where farmers usually obtain water from. These sites correspond to river water (22°26’3.45” S; 55°3’54.26” W), well water (22°27’8.79”S; 55°3’6.74” W), and weir water (22°29’8.42” S; 55°3’32.00” W). In addition, deionized water was used as a standard for comparison. Deionized water was produced by Outletlab equipment. The samples were put in 20 L thermoplastic boxes, labelled, immediately transported to the Physical-Chemical Laboratory, and stored in a refrigerated environment.
The following characteristics determined syrup quality: pH, electrical conductivity, turbidity, total dissolved solids, and total solids.
Water samples were separated into Beckers’s griffin cup type, with a 500 mL capacity. Half the volume (250 mL) was filled with water. Then, pesticides were added, and the container was completely filled with water, constituting the spraying syrup. The syrups were mixed and homogenized manually using a glass stick simulating the sprayer tank. After mixing, 4 repetitions were subdivided. Control treatments (without pesticide) and water types were put in scaled glass containers (beakers) and homogenized. Mineral oil was added only in fungicide treatments. Pesticides were added according to Abnt (2014).
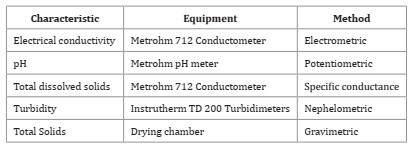
Physical-chemical characteristics of spray syrups associated with phytosanitary products were determined using standard methods 5, while equipment was tested as described in Table 4.
The physical-chemical data of spraying syrup were submitted to ANOVA, and the means were compared using the Tukey test, at 5% probability, Sisvar software 14.
Results and discussion
Significant interactions (p<0.05) were found between water qualities and product associations, regardless of sampling time. Total solids showed a significant influence of the tank-mixing multiple pesticides. Treatments with three classes of phytosanitary products (herbicide + insecticide + fungicide) presented the highest content of total solids, regardless of water quality throughout sampling times. The herbicide + fungicide and herbicide + insecticide + fungicide tank-mixing treatment also showed the highest concentration of solids in the off-season corn crop and with well water (Table 5, page 137).
Table 5: Water quality and pesticides association for total solids (mg L-1).
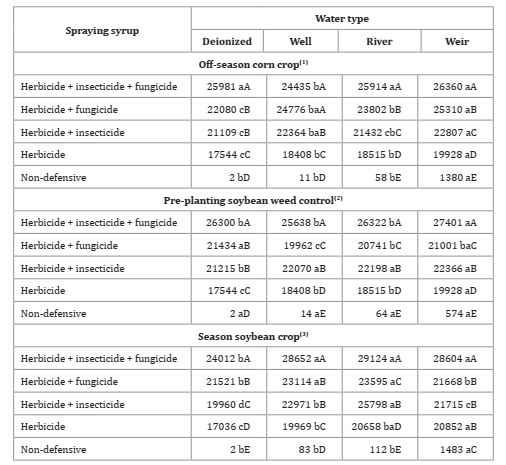
*F-test (p<0.05). Coefficient of variation = 2.88(1), 2.10(2) and 2.36(3)%. Different lowercase letters in the row and uppercase letters in the column indicate statistical for Tukey test, p ≤ 0.05.
*Prueba F (p<0,05). Coeficiente de variación = 2,88(1), 2,10(2)y 2,36(3)%. Diferentes letras minúsculas en la fila y mayúscula en la columna indican estadística para la prueba de Tukey, p ≤ 0,05.
In this sense, product association in the sprayer tank increased total solids, regardless of sampling times and water quality (Table 5). When phytosanitary products are mixed in the spray tank, physicochemical interactions given by unknown incompatibilities (two or more mixed chemicals suffer physical-chemical changes) may occur 37. This incompatibility can result in antagonistic effects and consequent reduced effectiveness of pesticides.
In control treatments, the highest total solids were found for weir water in the first and third seasons. However, no interaction was found between water qualities in the second sampling, probably given to absent rain (Figure 1, page 134). Similarly, water uptake influenced total solids in the spray syrup, also aggravated by low precipitation during the period. In the second period, all treatments with deionized water presented the lowest values for this variable, statistically differing from the other sources. Regardless of the season, a tendency for increased solids was observed in weir water treatments.
In the driest period, dissolved solids from drag dam and river edges resulted in greater concentration 2 generating greater turbidity. In the wettest period, these solids tended to be in lower concentrations. According to Farias et al. (2014), open-air sources, such as rivers and dams, are affected by rainwater and winds, carrying sediments such as clays. However, unlike weirs, rivers do not present still water, and thus, higher loading and dissolution of sediments reduced this water´s total solids values 37.
Solids in the syrup can inactivate glyphosate 20, which is adsorbed onto clay, reducing plant absorption and efficacy 24. Ramos and Durigan (1998) evaluated weir water influence on post-emergent herbicide efficacy. They found that solutions formulated with up to 10 g L-1 of soil from the Jaboticabal region containing 56% clay did not interfere with glyphosate efficacy. Brazilian soils are composed of low-activity clay, such as kaolinite. This means that cloudy water composition also impacts pesticide effectiveness. In tropical regions, such as the one in the present study, a higher concentration of organic matter (OM) in water, with greater effective cation exchange capacity, may result in greater adsorption of pesticides by OM than by clay minerals.
Analysis of total dissolved solids (Table 6) showed a precipitating tendency of products with the herbicide treatment, while the treatments with the association of three phytosanitary products (herbicide + insecticide + fungicide) showed lower dissolved solids between mixtures, regardless of water quality and sampling times analyzed, excepting well water at the off-season corn sampling.
Table 6: Water types and products association for total dissolved solids (mg L-1).
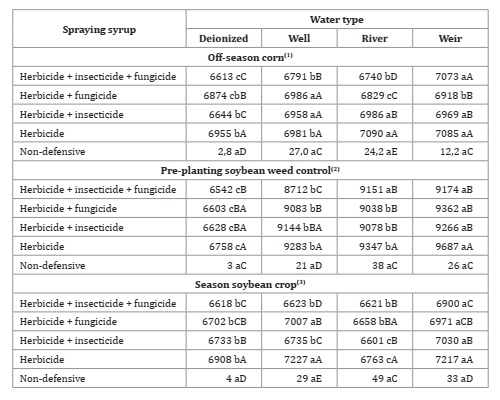
*F-test (p<0.05). Coefficient of variation = 0.56(1), 1.44(2) and 1.02(3)%. Different lowercase letters in the row and uppercase letters in the column indicate statistical for Tukey test, p ≤ 0.05.
*Prueba F (p<0,05). Coeficiente de variación = 0,56(1), 1,44(2) y 1.02(3)%. Diferentes letras minúsculas en la fila y mayúscula en la columna indican estadística para la prueba de Tukey, p ≤ 0,05.
These results indicate that when different phytosanitary products are associated, a higher content of solids in the sprayer tank may cause clogging of nozzles and filters.
Petter et al. (2012) and Pazini et al. (2013), evaluating association compatibility of different classes of phytosanitary products, concluded that depending on the added products, the association of more than one type of pesticide can cause physical incompatibility and low dissolution in the spray tank. According to these authors, this incompatibility is due to the type of formulation of phytosanitary products.
The concentrated suspension (SC) formulation is not always stable, and in resting syrup, solid particles can settle 19,37. When associating phytosanitary products with the same kind of formulation, there is hardly any incompatibility in the syrup. However, this rarely happens in the field. The treatment without any association (herbicide only) showed higher total dissolved solids, due to the high solubility of glyphosate in water (15,700 mg L-1 at 25°C and pH 7 - acid) 31, justifying the higher dissolved solid concentration in syrup.
Mean total dissolved solids obtained in the non-defensive treatments were similar between water qualities in the three sampling times (Table 6, page 138). Besides, dissolved solids alter water appearance, as turbidity 29. Both insecticide and fungicide increased spray syrup turbidity (Table 7).
Table 7: Water types and association of products for syrup turbidity.
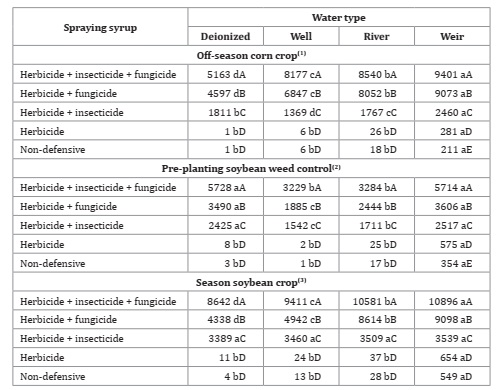
*F-test (p<0.05). Coefficient of variation = 0.45(1), 3.81(2) and 2.85(3)%. Different lowercase letters in the row and uppercase letters in the column indicate statistical for Tukey test, p ≤ 0.05.
*Prueba F (p<0,05). Coeficiente de variación = 0,45(1), 3,81(2) y 2,85(3)%. Diferentes letras minúsculas en la fila y mayúscula en la columna indican estadística para la prueba de Tukey, p ≤ 0,05.
Spray syrups made with weir water, including those treatments without pesticides, were the ones with the highest turbidity values, regardless of product association. As already mentioned, water quality for agricultural spraying is closely related to spray physical quality considered as suspended sediment content. When analyzing waters without pesticides in all sampling periods, higher concentrations of dissolved solids were observed in weir water, representing a greater potential for interaction with pesticides, and consequent less effectiveness.
Treatments with only herbicide did not differ from the treatments without pesticides (water only) regarding turbidity, except for the herbicide treatment with weir water in the first and second sampling times. Considering turbidity may be influenced by product formulation, soluble formulations such as glyphosate, highly soluble in water 4, generate lower turbidity. In contrast, concentrated suspension formulation (fungicide and insecticide) with a milky appearance, increase syrup turbidity (Photo, page 140).

Foto: Turbidimetría de los productos fitosanitarios en agua desionizada. T1: Herbicida; T2: Herbicida + Insecticida; T3: Herbicida + Fungicida; T4: Herbicida + Insecticida + Fungicida.
When observing mixtures of the herbicide lactofen (Drible® 240 - formulation CE - emulsifiable concentrate) with the insecticides Methomy Chlorpyrifos, Cypermethrin, Thiamethoxam/Lambdacialotrine, Teflubenzuron, and Triflumurom, Petter et al. (2012) found physical incompatibility in syrups prepared with water, water with pylyrolean acid and water with boric acid. This incompatibility varied between grade 2 (separation after 1 minute, not to be applied) and grade 4 (separation after 10 minutes, apply on continuous agitation), resulting in non-homogeneous mixing, with a decanting tendency. Before, Petter et al. (2007) had observed physical-chemical interaction caused by CE with Chlorpyrifos SC (flowable suspension concentrate). According to Theisen and Ruedell (2004), most physical and chemical incompatibilities are observed in mixtures of products with CE formulations and WP (Wettable Powders), EW (emulsion in water) and SC.
In this study, product association altered spraying syrup pH (Table 8).
Table 8: Water types and products association for pH of spraying syrup.
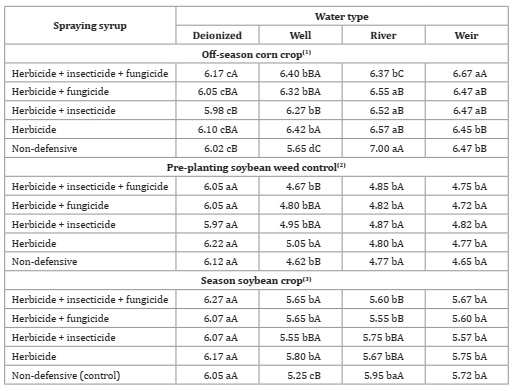
*F-test (p<0.05). Coefficient of variation = 1.03(1), 3.34(2) and 2.82(3)%. Different lowercase letters in the row and uppercase letters in the column indicate statistical for Tukey test, p ≤ 0.05.
*Prueba F (p<0,05). Coeficiente devariación= 1,03(1), 3,34(2) y 2,82(3) %. Diferentes letras minúsculas en la fila y mayúscula en la columna indican estadística para la prueba de Tukey, p ≤ 0,05.
This variation was lower in the soybean pre-planting weed control sampling. Treatments with phytosanitary products did not differ from each other, except for well water. However, no abrupt change between any treatments occurred, nor did they present alkaline pH (pH> 7), which according to Vuković et al. (2013), causes syrup instability for acidic products.
During pre-plating soybean weed control and soybean crop, no statistical difference between treatments was observed for deionized and well water, i.e., product addition did not influence spray pH (Table 8, page 140). Deionized water is pure water that has undergone a process of filtration and total removal of ions like nitrate, Calcium, and Magnesium, among other elements 32. This is considered ideal for spray syrup water, precisely for keeping water quality indicators, such as pH 19. On the other hand, the non-influence of weir water on pH is related to a higher concentration of suspended particles than ions, thus affecting attributes like dissolved solids and solids before affecting pH. For well water, during the three sampling times, an increase in pH was observed after the addition of phytosanitary products, except for the association between herbicide + insecticide + fungicide, in the soybean pre-planting weed control sampling, the driest period. Another relevant fact is that, regardless of sampling time, when glyphosate was added to well water, the highest pH increase was observed. Typically, well waters have high ion concentrations, especially: Ca 2+, Mg 2+, Na+, K+, HCO3 -, CaSO4 2-.
Well waters are often classified as hard waters, with high CaCO3 concentrations. Variations in average hardness are related to soil geological nature. Due to high CaCO3 concentration, well water usually reports alkaline pH 7,36,39. The use of this hard water may cause ion chelation by glyphosate 34, decreasing efficiency. In addition, given well water alkalinity and glyphosate acid nature, this herbicide is dissociated, losing efficiency 30. Additionally, after mixing phytosanitary products, the resulting syrup may present different pH values. In general, association incompatibility occurs with high syrup pH alteration, leading to degradation of active ingredients and consequent undesirable chemical compounds 37.
Water pH IS The main parameter influencing spray syrup compatibility 11. Ideal pH for plant protection varies among products 37. Pyrethroids insecticides have a pH efficiency of around 4. Carvalho et al. (2009) report syrup pH ranging from 4.6 to 5 for glyphosate, while Schilder (2008) mentions 5 to 6. Cunha et al. (2017) observed that the combination of Trifloxystrobin + Prothioconazole had pH 7, while with the adjuvant soy methyl ester, achieved pH 5. Most phytosanitary products, in the presence of alkaline pH (>7), decompose rapidly causing in-tank associations instability 17.
Syrup mean electrical conductivities (EC) differed among all treatments (Table 9, page 142).
Table 9: Water types and products association for electrical conductivity (μS cm-1).
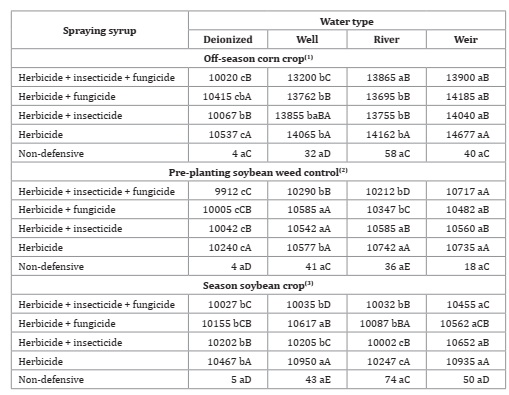
* F-test (p<0.05). Coefficient of variation = 1.38(1), 0.76(2) and 1,02(3)%. Different lowercase letters in the row and uppercase letters in the column indicate statistical for Tukey test, p ≤ 0.05.
*Prueba F (p<0,05). Coeficiente de variación = 1,38(1), 0,76(2) y 1,02(3)%. Diferentes letras minúsculas en la fila y mayúscula en la columna indican estadística para la prueba de Tukey, p ≤ 0,05.
Glyphosate highly influenced spray syrup EC. The more acidic the water, the greater the electrical conductivity measured in the syrup after glyphosate addition. According to Amarante Júnior et al. (2002), glyphosate is a strong acid with a pH between 2.2 and 5.4, with two dissociations, and a pH 5.5 with three dissociations and an increasing EC.
Evaluating surface tension, pH, and EC of phytosanitary syrup, Cunha et al. (2017) demonstrated that glyphosate presented the highest conductivity among tested products using deionized water, evidencing that water addition influences spray syrup EC. In this study, glyphosate EC increased at pH above 6.3. Similarly, with the tank-mixing multiple pesticides, a decrease in syrup EC was observed when compared with the treatment without association, proving that tank-mixing causes chemical interaction between the products 39. Thus, decreasing EC was measured in the three sampling times and with distinct pH ranges between seasons.
Regarding non-defensive treatments, no difference was found between water qualities at any time of analysis. These results differed from those found by Rheinheimer and Souza (2000) and Farias et al. (2014) when verifying low EC values in weir water and higher in well water.
Electrical conductivity represents the solution ion concentration that can conduct electricity. Higher conductivity values express higher number of ions in the solution. Thus, the greater the possibility of these ions interacting with agrochemical molecules, the greater the incompatibility of the syrup 8. However, there is no default value for EC, as this characteristic depends on the application volume. According to Vargas et al. (1997), smaller application volumes cause less EC influence on herbicides. i.e., decreasing ion proportion concerning pesticide molecules. Considering the same concentration, using an application rate of 1.0 L ha-1, allows lower ion interference (EC) than an application using 30 L ha-1 (6.
In this context, this study showed a tendency to increase product dissolution in the spray syrup for no products association and when using high quality water, such as deionized water.
Phytosanitary product solubility is measured as solubility in water, variable among active ingredients. Glyphosate had the highest water solubility among tested active ingredients by Soares et al. (2017). For this reason, correct syrup preparation and association of phytosanitary products in the spray tank, are important 19. However, even following preparation order, treatments associated with tank-mixing multiple pesticides presented worse physical-chemical quality.
Conclusions
Water from different sources and tank-mixing multiple pesticides affect spraying syrup quality. Weir water presents the worst physical quality among the tested sources. It may decrease pesticide performance.
Spraying syrup prepared with tank-mixing herbicide, insecticide, and fungicide in various combinations increased in the successive cultivation of soybean/corn in the studied area. When the three pesticides are associated, less dissolution of the spray syrup and a greater risk of syrup incompatibility takes place, rising fungicide ineffectiveness.