Introduction
Tocopherols and Selenium are important antioxidants that inhibit lipid peroxidation and free radical formation, fighting cell damage and preventing cancer and heart diseases 25. Selenium, a crucial trace element, is an important constituent of several selenoproteins. It regulates the synthesis of certain key antioxidant enzymes called glutathiones. Among these enzymes, the widely studied glutathione peroxidase (GSH-px), detoxifies peroxides and hyperperoxides. Further, Sodium selenite and selenomethionine (an organic form that can be biologically metabolized by animals), constitute a principal sources of Selenium 23.
Vitamin E comprises several compounds called tocopherols. Alpha-tocopherols have important activity in organic antioxidant protection, mainly preventing peroxidation processes of long cell membrane-chain fatty acids 19. In Japanese quails, Akil and Piliang (2012) observed that increasing deposition of tocopherol and Selenium in egg yolk was directly proportional to dietary supplementation levels of these compounds. Additionally, they also discovered a reduction in yolk concentration of malondialdehyde during supplementation of Selenium in combination with vitamin E, thereby causing an increase in hatchability. Conceptually, foods enriched with bioactive compounds are called functional foods or nutraceuticals 11. Due to the innumerable and well-known advantages of quail production, biofortified eggs can be an important functional food. Thus, this study aimed to evaluate the nutraceutical effects of supplementing organic Selenium and vitamin E on performance, egg quality and antioxidant protection of Japanese quails.
Material and Methods
The trial was carried out at the Experimental Poultry House, located in the State of Rio de Janeiro- Brazil, latitude 22°51’08” S, longitude: 43°46’31” W and 13 m a. s. l. A total of 252 Japanese Fujikura female quails with an average weight of 188.32 ± 4 grams and a mean stance of 90%, were utilized. The experiment was approved by the Institution’s Ethics and Biosafety Committee (number 0059/2013).
Lot standardization was subsequently followed by quail random distribution in 42 posture cages (dimensions of 33 x 25 x 20 cm). The experimental design was entirely randomized with seven treatments, six replicates and six birds per cage. The treatments consisted of the addition of 200 mg of vitamin E/kg of feed (DL-α-tocopheryl acetate 99%) and increasing levels of organic Selenium. The experimental rations were obtained from a control diet, with increasing levels of organic Selenium (0.10, 0.20, 0.30, and 0.40 mg of selenomethionine per kilogram of feed) and 200 mg of acetate of DL-alphatocopheryl per kg of diet (as a source of vitamin E). Treatments were as follows: (1) Control diet (CD); (2) CD + 200 mg of vitamin E (VE); (3) CD + 0.20 ppm Selenium yeast (SE); (4) CD + 0.10 ppm Selenium yeast + 200 mg vitamin E (SVE1); (5) CD + 0.20 ppm Selenium yeast + 200 mg vitamin E (SVE2); (6) CD + 0.30 ppm of Selenium yeast + 200 mg of vitamin E (SVE3); (7) CD + 0.40 ppm Selenium yeast + 200 mg vitamin E (SVE4).
According to the nutritional requirements of Japanese quails (16), diets were calculated by Super Crac 5.0 TD package, except for crude protein and calcium, which were based on the recommendations of Oliveira et al. (1999) and Barreto et al. (2007), respectively. Then, the values of apparent metabolizable energy of corn, soybean meal, and soybean oil were adjusted according to Moura et al. (2010). Table 1 shows experimental dietary composition and vitamin and mineral supplementation.
Performance variables were mean egg production (%/quail/day), feed consumption (g/quail/day), egg weight (g), egg mass (quail/day), and feed.
For egg quality assessment (a) yolk weight (g); (b) albumen weight (g); (c) shell weight (g); (d) percentage of yolk; (e) percentage of shell; and (f) percentage of albumen, were evaluated. Tocopherol and Selenium nutritional effects were evaluated 42 days after supple mentation, analytically determining nutrient concentrations. Metabolic indicatorsmalondi aldehyde (MDA) in quail egg and blood were determined, according to Shahryar et al. (2010) and Enkvetchakul et al. (1995): (a) concentration of total yolk tocopherols (μg/g egg); (b) egg bark Selenium, (μg/g), and blood (μg/l); (c) blood MDA (mmol/l) and egg yolk MDA (mmol/g); (d) blood GSH-px (EC 1.11.1) (unit/l).
For further evaluation of Selenium/vitamin E supplementation effects on egg antioxidant protection and consequent shelf life, 36 samples per replicate were stored up to 24 days at 22°C. For this purpose, firstly, all quails were fed with a reference adaptive ration for seven days. Subsequently, they were offered the respective ad libitum experimental rations. Yolk MDA was analyzed every 8 days (1, 8, 16 and 24 days). In addition, the birds were submitted to a photoperiod of 17 hours. Daily temperature and relative humidity were recorded. Feed intake, egg production, weight and mass were measured weekly.
Yolk, albumen, and bark weight were determined by collecting three eggs from each replicate, daily. As a reference, on the experimental “zero” day, 80 eggs were randomly collected and evaluated under the same protocol. In the last four days of the trial, 12 eggs of each replicate were collected and sent to the laboratory for Selenium and vitamin E deter minations. For micronutrients analysis, high-performance liquid chromatography (HPLC) was employed, according to Marques et al. (2011).
At the end of the trial, three quail blood samples per repetition were collected via the brachial vein. Subsequently, free Selenium, MDA, and glutathione peroxidase (GSH-px) plasma concentrations were determined. Statistical analysis included ANOVA, composite symmetry with a constant correlation between repeated measures over time; autoregressive correlations between repeated measures over time; and variance/covariance unrestricted structure. Model fit was performed using PROC MIXED (SAS System, Inc., Cary, NC, USA).
Performance variables were analyzed by mixed models PROC MIXED, (SAS System, Inc., Cary, NC, USA), and Tukey test. Egg quality, vitamin E and Selenium were analyzed by generalized linear models, using GLIMMIX (SAS System, Inc., Cary, NC, USA), and Tukey test. The mathematical model adopted for mathematical procedures was:
in which = observation of Yij is the j-th treatment in the i-th observation, μ = overall mean, aj= effect of experimental diet level j, and eij = random error for each observation Yij.
Results
For performance variables, only egg production, feed intake, and egg mass showed significant differences (p < 0.05) between treatments. The supplementation of 200 mg of Vit E + 0.40 ppm of organic Selenium in the diet reduced feed intake, probably causing the evidenced loss in egg production and mass (Table 2).
No significant difference (p > 0.05) was observed in egg quality (Table 3).
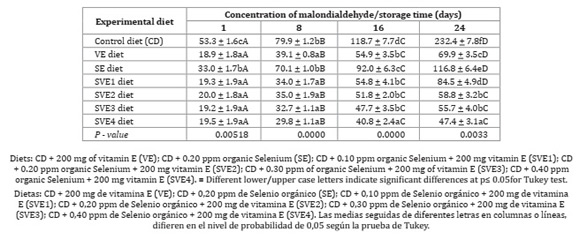
Significant difference (P <0.05) was found for vitamin E supplementation on α-Tocopherol deposition, as well as in GSH-px activity and MDA oxidative bioindicator, as described in Table 4, Figure 1 and Figure 2 (page 150).
Table 4: Alpha-tocopherols, Selenium, and malondialdehyde concentration, and glutathione activity in egg and blood of Japanese quails.
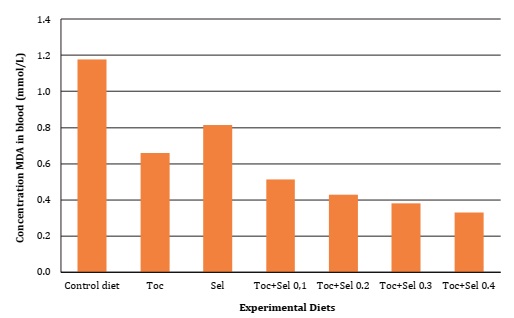
Figura 1: Malondialdehído en sangre de codorniz en función de la suplementación con vitamina E y Selenio.
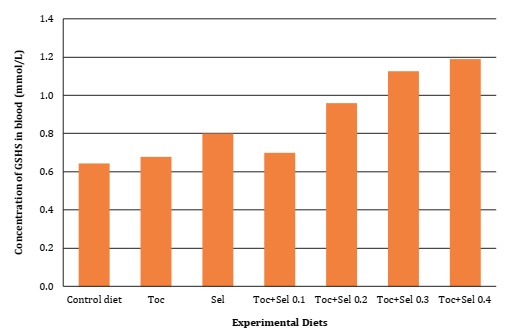
Figura 2: Concentración de Glutatión peroxidasa reducida (GSH-px) en sangre de codorniz en función de la suplementación con vitamina E y Selenio.
Adding vitamin E and Selenium, extended shelf life of quail eggs (Table 5, page 150).
Discussion
These results indicate a possible toxic effect of Selenium 6 on quail folliculogenesis, increasing the production of infertile eggs, as also studied by Oldfield (1987), Spallholz & Hoffman (2002) and Fairbrother et al. (2012).
Contrarily, Canogullari et al. (2010) reported no significant differences in feed intake, egg yield and egg weight. They explained that organic Selenium supplementation was more effective than inorganic supplementation, at increasing egg Selenium content. According to Liu et al. (2020), adding 0.5 mg/kg of dietary Selenium significantly improved egg production performance of hens, while organic supplementation allowed higher yolk Selenium than inorganic Selenium. These results corroborate those scientific results on nutritional requirements and the interactions of micronutrients with laying quails during their early stages. Our results agreed with Mori et al. (2003), who verified the linear relationship between the concentration of tocopheryl acetate supplemented in chicken diets and yolk α- tocopherol content. As evidenced in the present research, Selenium supplementation affected egg α-tocopherol concentration while the interaction with vitamin E significantly increased GSH-px and MDA concentrations (Table 4, page 149).
According to Shah et al. (2016), the concentration of glutathione peroxidase and superoxide increased when vitamin E reached 100 and 150 mg/kg in the feed. Antioxidant vitamins have an inverse relationship with lipid peroxidation inside the body, resulting in cell damage. Vitamin E is a natural antioxidant with an essential role against lipid peroxidation 9. Jena et al. (2013) reported that superoxide dismutase (SOD) and catalase activity increased significantly when broilers were supplemented with vitamin E under heat stress conditions. Previously, Özkan et al. (2007) found that at low temperatures (14.5 to 16.8°C), supplementation of vitamin E in combination with Selenium enhanced broiler liver glutathione peroxidase. Behavioral stress produces free radicals and ROS, which can potentially harm the cell membrane by peroxidation of polyunsaturated fatty acids 10.
As for vitamin E as a function of storage time, yolk malondialdehyde also exhibited a significant difference (p < 0.05). Adding dietary vitamin E and/or Selenium increased egg shelf life with a synergistic effect, which replied to the increase of organic Selenium (Table 5, page 150). Baylan et al. (2011) explained that a high level of Selenium -yeast administration to quail diet increased storage time compared to selenite and control groups.
Vitamin E and Selenium supplementation promoted lower yolk MDA concentration over time (Table 4, page 149). However, the associated supplementation of alpha-tocopherol with organic Selenium exhibited higher efficiency in inhibiting lipid peroxidation, evidencing, once more, the synergistic effect of micronutrients when compared to isolated supplementation. These results coincide with Surai (2002) and Akil and Piliang (2012), who reported a synergistic advantage of tocopherols and Selenium protection against oxidative stress and prevention of peroxidation of long-chain fatty acids. Surai and Dvorska (2002) evaluated GSH-px, vitamin E and Selenium activity in stored eggs, showing that combining vitamin E and Selenium was more efficient against lipid peroxidation during storage time.
Conclusion
The supplementation of 200 mg of α-tocopherol and 0.30 ppm of organic Selenium decreased feed intake but did not affect other performance parameters. Egg quality was not influenced by dietary vitamin E and/or Selenium. The synergistic effect of including α- tocopherol and organic Selenium on malondialdehyde levels and glutathione activity was statistically significant especially when the mineral supplementation was increased.
Egg shelf life increased with α-tocopherol and increasing levels of organic Selenium, confirmed by the lower production of malondialdehyde.