Introduction
The Cuyo region in Argentina is an arid zone with heterogeneous and poorly structured soils with low fertility and organic matter. Thus, performing management tasks favoring higher biological activity and nutrient availability turns necessary for sustainable crop growing 1. Moreover, due to the limited information about organic amendments in these arid soils, several studies suggest an increase in crop productivity and improvement in soil properties by bio-input applications 20.
Worldwide, tomato (Solanum lycopersicum L) and lettuce (Lactuca sativa) are two important horticultural crops, being tomato the second most consumed in Argentina. These crops are mainly produced in oases located in arid zones, being Cuyo region the highest cultivated surface 35, with Mendoza province as the main tomato producer with 3,757 ha, and 265 ha for lettuce 16. However, the intensive farming of tomatoes and lettuce requires a large amount of high-cost synthetic agrochemicals to cover nutrient requirements and control diseases and plagues, thus having negative environmental impacts 5,9.
Therefore, organic products are becoming a cheaper and low-impact alternative to synthetic ones. In this sense, biopesticides and biofertilizers developed locally represent a viable alternative for sustainable crop management, lowering environmental negative effects and production costs. Bioproducts are biologically active supplies that promote plant growth by different strategies 10, such as increasing nutrient availability, producing phytohormones and antibiotics, and/or competing against pathogens 26,27. Furthermore, natural compounds may quickly biodegrade, due to microbiologic coevolution and decomposition metabolic pathways 7.
Bioslurry or digestate, obtained by anaerobic digestions of fresh organic matter, presents adequate characteristics for plant growth. Most of the available bibliography refers to bioslurry as the byproduct or residue of biogas production with biofertilizer and biocontroller properties against plant diseases 8,17. The liquid fraction derived from anaerobic digestions can retain nutrients and microorganisms. It has also been tested as biofertilizer, soil amendment, and even for bioremediation, with promising results 29,30,36.
By-products of anaerobic digestions have been also developed as plant biostimulants, with no biogas generation. The Food and Agriculture Organization (FAO), proposed a protocol to obtain a bioslurry specifically designed for plant nutrition and growth promotion, based on elemental and microbial content in the final product 12. Other authors have reported lactic fermentation for obtaining adequate biostimulants for plant growth, showing promising results 22. Nevertheless, there is a lack of information about the properties of these non-methanogenic bioslurries in plant nutrition and antipathogen effectiveness.
The study evaluated three different bioslurries obtained from different raw materials and elaboration processes, as biofertilizers for tomato and lettuce. The hypothesis stated that anaerobic digestions generate by-products rich in nutrients and microorganisms that increase plant development.
Methods
Digestates elaboration
Three different digestates or bioslurries were tested as plant biostimulants: bioslurry obtained from biogas digestion (Biog); bioslurry designed for plant nutrition by FAO (Bfao); and a lactic fermentation lixiviate (Blac). Biog was obtained from biogas digestion of a traditional mixture of water:goat manure in a 9:1 ratio. The biodigester was built with two 200 μm polyethylene layers in a 1.25 m3 tubular design intended to be semi-buried in cold climates, for integrated solar gain systems and insulated enclosure. During Biog production, the biodigester was operated at 25 °C with a hydraulic retention time of 60 days and fed twice a week with a 7 kg load of goat manure and 60 kg of water. Bfao was brewed according to FAO protocol (2013): An anaerobic process was carried out in a 200 L PET recipient, with a screw cap and a gas trap containing 10 kg of fresh vegetal material, 60 L of fresh goat manure, 3 kg of wood ashes, 4 kg of bentonite, 500 g of chicken eggshells, 3 kg of bone ashes, 5 L of cow milk, and free chloride water to a final volume of 170 L. After 3 months of storage, the product was filtered and stored in darkness at 15-20 °C. Finally, Blac elaboration protocol was based on Quirós et al. (2004): a mixture of 1 L of commercial rice and 2 L of chlorine-free water was left to settle for 48 h. Later, the lixiviate obtained was supplemented with 6 L of milk and stored at 30 °C for 3 days. Finally, it was filtered and stored until use.
Bioslurries characterization
Toxicity test
Lactuca sativa var Grandrapids seeds were used for toxicology tests according to US EPA (1996). Seeds were previously tested for germination power and seed viability using sterile distilled water. Then, seeds were superficially sterilized with 70% ethanol and exposed to the biostimulants as follows: 20 seeds were placed on a filter paper in 90 mm Petri dishes, exposed to 0, 10, 25, 50, and 100% dilutions of each product by triplicate, and maintained at 20 °C in darkness for 5 days. Control treatment and dilutions were performed with well water (H2O), also used for product brewing. To evaluate the toxic effect, total germination, and hypocotyl/root elongation, were analyzed.
Physico-chemical analyses
To perform a basic characterization, pH, CE, and macronutrients were analyzed in each bioproduct by duplicate. Total nitrogen (N) was determined in dry samples by Kjeldahl and steam trawl distillation method 4. Phosphorus (P) was colorimetrically analyzed by HCl extraction with ammonium methavanadate, ammonium molybdate, and nitric acid solution. Absorbance at 420 nm was measured with a UV-VIS Milton Roy spectrophotometer. Finally, K was determined by flame atomic absorption spectrophotometry 32.
Pot assays
To evaluate bioslurries effect on plant growth, seeds of Lactuca sativa var Grand rapids and Solanum lycopersicum var Platense were germinated and grown in seedling trays for 15 days, with basal fertilization of 500 mg of commercial fertilizer (KSC® 2 NPK 23-5-5, Timac Agro USA). Seedlings were transplanted into a 0.5 L pot containing perlite:peat (1:1) and grown under greenhouse conditions (23±2 °C, 30% humidity, and natural 16/8 h photoperiod due summer season). All plants were irrigated with well water every 48 h to maintain field capacity during the assay. After transplant, homogeneous 10 cm plants were selected for treatment initiation. A complete randomized block design was established with 5 treatments for each plant species (n=8): Biog, bioslurry from biogas production; Bfao, bioslurry designed for plant nutrition by FAO (2013); Blac, lixiviate of a lactic fermentation; Cont, well water; and Fert, chemical fertilization. Throughout the assay, 50 mL of each product diluted at 10% were weekly applied.
Aerial and root dry weight (DWa, DWr, respectively) were determined in 120 days old plants. Additionally, yield (Fv/Fm) as an indicator of photosystem II damage, and performance index (PIabs) as stress resistance capacity, were measured with a Chlorophyll Fluorimeter Handy Pocket PEA (Hansatech Instruments Ltd., King’s Lynn, Norfolk, England). This was carried out with a leaf-clip placed on the third leaf from the apex for 20 min till dark adaptation 13.
Finally, chlorophyll content index (CCI) by absorbance was also determined in the third leaf with a chlorophyll meter (model Clorofilio, Cavadevices, Argentina).
Statistical analysis
ANOVA was performed considering the block design, and a LSD Fisher means comparison test (p<0.05) evaluated the effect of the digestates on seedling biomass. Shapiro-Wilk normality tests and residues regression were carried out to confirm ANOVA assumptions. Due to the lack of normality, phytotoxicity test was analyzed by non-parametric Kruskall Wallis test (p<0.05). InfoStat software version 2015 performed all statistical analyses (InfoStat Group, FCA, National University of Córdoba, Argentina). All data was expressed as mean ± standard deviation.
Results
Phytotoxicity test
Blac treatment decreased seed germination at concentrations of 100, 50, and 25%, while 10% did not differentiate from control even though the value was lower. Biog and Bfao did not show significant differences from control in any dilutions, except for Biog 100%, with zero germinated seeds.
Root and hypocotyl elongation was negatively affected by Blac in all concentrations, whereas Bfao and Biog 10% significantly increased these parameters. At 25%, both Bfao and Biog significantly increased plant hypocotyl while not affecting root elongation. At higher concentrations, all products showed toxicity, reducing plant elongation (Table 1, page 52).
Table 1: Phytotoxic effect of bioslurries on Lactuca sativa var Grand Rapids seeds. Tabla 1: Efecto de fitotoxicidad de los bioles en semillas de Lactuca sativa var Grand Rapids

Kruskal Wallis p= 0.05. Values are expressed as mean ±SD.
Se realizó un análisis de Kruskal Wallis con una significancia del 0,05. Valores expresados como media ±DS.
Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); Blac: lixiviate of a lactic fermentation; and H2Od: distilled water used as control.
Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica; y H2Od: agua destilada usada como control.
Except for Blac, no treatment differed from control at 10%, suggesting no phytotoxicity properties or plant growth stimulation. Such concentration was considered for further analyses based on these results and the bibliography. Blac 10% was included in further assays for results verification, considering that in vitro conclusions may be limited and different results may be expected in pot treatments.
Bioslurries characterization
Only Bfao presented an adequate pH value (Resolución 19/2019, Secretaría de Gobierno de Ambiente y Desarrollo Sustentable, Argentina; Ministry of Environment and sustainable development of Argentina). All products presented high EC levels, while Blac also presented high acidity (low pH value). Considering the phytotoxicity results, all products must be diluted, being 10% the most adequate dilution (Table 2, page 52).
Table 2: Bioslurries physico-chemical characterization. Each product was analyzed by duplicate. Tabla 2: Caracterización fisicoquímica de los bioles. Cada producto fue analizado por duplicado.

Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); and Blac: lixiviate of a lactic fermentation.
Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica.
Effect of bioslurries on lettuce growth
There were no significant differences among biolsurries treatments. Nevertheless, Bfao and Blac showed significantly higher aerial biomass (2.17 ±0.54 and 2.33 ±1.13 g respec tively), related to control (1.16 ±0.60 g; Figure 1B, page 53).

Cont: control, irrigated with water; Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); Blac: lixiviate of a lactic fermentation; and Fert: inorganic fertilizer (KSC® 2 NPK 23-5-5, Timac Agro USA). Cont: control, regado con agua; Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica; y Fert: suplementado con fertilizante inorgánico (KSC® 2 NPK 23-5-5, Timac Agro USA). Values are expressed as mean ±SD. ANOVA (LSD Fischer, p<0.05). Valores expresados como media ±DS. ANOVA (LSD Fischer, p<0,05)
Figure 1: Effect of bioslurries on Lactuca sativa total (A), aerial (B) and root biomass (C). Figura 1:Efecto de los bioles en la biomasa total (A), aérea (B) y radical (Ca) de Lactuca sativa.
Root biomass was only increased by Bfao (1.60 ±0.44 g) (0.66 ±0.34 g; Figure 1C, page 53). As expected, Fert was the treatment with significantly higher values of plant biomass (3.64 ±1.21 and 6.2 ±1.50 g, respectively for root and aerial dry weight, Figure 1A, page 53).
CCI in lettuce plants was significantly increased by all digestates with respect to control (18.61 ±3.35), with the maximum value reached by Fert treatment (35.06 ±2.00), and followed by Bfao (30.59 ±3.70). Biog (23.33 ±1.27) and Blac (24.17 ±2.12) showed lower values and did not differentiate from each other (Figure 2A, page 54).
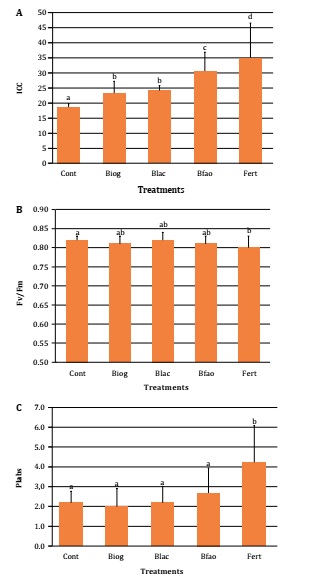
A: CCI: chlorophyll content index; B: Fv/Fm: yield index, indicators of photo system II damage; and C: PIabs: performance index, indicator stress resistance capacity. Cont: control, irrigated with water; Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); Blac: lixiviate of a lactic fermentation; and Fert: inorganic fertilizer (KSC® 2 NPK 23-5-5, Timac Agro USA). A: CCI: índice de contenido de clorofila; B: Fv/Fm: índice de rendimiento, indicador de daño del fotosistema II; y C: PIabs: índice de desempeño, indicador de la capacidad de resistir estrés. Cont: control, regado con agua; Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica; y Fert: suplementado con fertilizante inorgánico (KSC® 2 NPK 23-5-5, Timac Agro USA). Values are expressed as mean ±SD. ANOVA (LSD Fischer, p<0.05). Valores expresados como media ±DS. ANOVA (LSD Fischer, p<0,05).
Figure 2: Effect of bioslurries on nutritional and stress indicators in Lactuca sativa. Figura 2:Efecto de los bioinsumos en indicadores nutricionales y de estrés en Lactuca sativa.
As stress indicator, damage in photosystem II was significantly higher in Fert (0.80 ±0.03), regarding Cont (0.82 ±0.01), reaching the lowest Fv/Fm value, while Fert and Cont digestates did not differentiate (Figure 2B, page 54). Oppositely, PIabs were significantly increased by Fert (4.23 ±1.85), and digestates did not differentiate from Cont (2.19 ±0.58), with Bfao showing the highest value (2.68 ±1.26, Figure 2C, page 54).
Effect of bioslurries on tomato growth
Bioslurries did not significantly increase root dry biomass in tomato plants, and were significantly lower than Fert treatment. As in lettuce, Fert was the treatment with the highest root and aerial biomass (33.53 ±11.45 and 83.09 ±5.39 g for) while control showed the lowest biomass values (Figure 3, page 55).

Cont: control, irrigated with water; Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); Blac: lixiviate of a lactic fermentation; and Fert: inorganic fertilizer (KSC® 2 NPK 23-5-5, Timac Agro USA). Cont: control, regado con agua; Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica; Fert: suplementado con fertilizante inorgánico (KSC® 2 NPK 23-5-5, Timac Agro USA). Values are expressed as mean ±SD. ANOVA (LSD Fischer, p<0.05). Valores expresados como media ±DS. ANOVA (LSD Fischer, p<0,05). ).
Figure 3: Effect of bioslurries on Solanum lycopersicum total (A), aerial (B) and root biomass (C) Figura 3: Efecto de los bioles en la biomasa total (A) aérea (B) y radical (C) de Solanum lycopersicum.
Aerial biomass was significantly increased only by Bfao (10.21 ±3.05 g), in relation to Cont (5.47 ±1.38 g), while Blac and Biog did not show significant effects.
All digestates significantly increased CCI in tomato plants compared to control (22.1 ±3.56). The maximum value was reached by Fert treatment (42.66 ±3.44), followed by Bfao (37.69 ±3.71). As in lettuce plants, Biog (29.04 ±6.55) and Blac (27.5 ±4.16) did not differentiate from each other and presented lower values (Figure 4A).

A: CCI: chlorophyll content index; B: Fv/Fm: yield index, indicators of photosystem II damage; and C: PIabs: performance index, indicator stress resistance capacity. Cont: control, irrigated with water; Biog: bioslurry from biogas production; Bfao: bioslurry designed for plant nutrition by FAO (2013); Blac: lixiviate of a lactic fermentation; and Fert: inorganic fertilizer (KSC® 2 NPK 23-5-5, Timac Agro USA). A: CCI: índice de contenido de clorofila; B: Fv/Fm: índice de rendimiento, indicador de daño del fotosistema II; y C: PIabs: índice de desempeño, indicador de la capacidad de resistir estrés. Cont: control, regado con agua; Biog: biol proveniente de la producción de biogás; Bfao: biol diseñado para la nutrición vegetal, en base a FAO (2013); Blac: lixiviado de una fermentación láctica; y Fert: suplementado con fertilizante inorgánico (KSC® 2 NPK 23-5-5, Timac Agro USA). Values are expressed as mean ±SD. ANOVA (LSD Fischer, p<0.05). Valores expresados como media ±DS. ANOVA (LSD Fischer, p<0,05).
Figure 4: Effect of bioslurries on nutritional and stress indicators in Solanum lycopersicum. Figura 4: Efecto de los bioinsumos en indicadores nutricionales y de estrés en Solanum lycopersicum.
Yield index Fv/Fm was significantly higher in Bfao (0.70 ±0.02), Blac (0.74 ±0.02) and Fert (0.74 ±0.08), with respect to Cont (0.64 ±0.13), and with no differences among them (Figure 4B). PIabs was significantly increased only by Fert (1.57 ±1.55). Nevertheless, no differences were detected among digestates and Cont treatments (Figure 4C).
Discussion
In the present study, we demonstrated the ability of different bioslurries or digestates obtained from organic waste as biofertilizers, resulting in high-quality inputs for agricultural production. Generally, digestates are the result of anaerobic digestion of organic residues for energetic generation, suggesting the potentiality of anaerobic processes to reduce negative environmental impacts.
However, digestates may have high EC, exceeding the 3 dS m-1, limit established for irrigation water, and potentially toxic for agriculture production 24. It may also contain high ammonia concentrations causing decreased oxygen concentration in the root system 6. According to our results, the retention time of each elaboration process seems independent to element solubilization. Bfao, with a retention time of 90 days, did not show higher NPK values than a 60 day Biog, while Blac showed the highest N and P content, with 5 days of brewing time.
In line with other studies, for plant growth, dilutions are commonly needed to reduce phytotoxic effects of pure products. In agriculture, there is no agreement among authors on the optimal dilution of digestates for maximum stimulation and minimum toxicity. Song et al. (2021) reported 20% as digestate optimal concentration for biostimulant usage in several horticultural crops. Nonetheless, Díaz Montoya (2017) suggested negative effects on lettuce germination at concentrations above 2-4%; and Silva et al. (2011) reported possible phytotoxicity at concentrations higher than 10%. Our results indicated dilutions lower than 25% for Bfao and Biog, and 10% for Blac. This suggests a high influence of the raw material used for bio-inputs brewing, determining the quality and variety of the nutrient and metabolites, more than the EC itself. Despite the higher EC of Bfao and Biog, lettuce plants showed low toxicity at a higher digestate concentration (25%), while in Blac, a 10% dilution was necessary, avoiding negative effects.
Tomato and lettuce biomass increased with the bioproducts, mainly by Bfao, due to its specific design for plant growth. Biog seemed to stimulate growth but not significantly from control, while Blac significantly increased lettuce biomass. Regardless of the high N and P content of Blac, its elaboration process is focused on lactic bacteria content and their effect on plant growth. These microorganisms serve as biofertilizers, biocontrollers, biostimulants, and bioelicitors 19,33, probably explaining the increase in lettuce biomass. On the other hand, Biog process is the only one not trying to increase plant nutrition and beneficial microorganisms, but producing energetic compounds. However, several studies have reported this product´s biostimulant quality 3,15. Accordingly, our results displayed an increase in plant biomass, showing no toxic effects at dilutions below 25%.
Inhibition of plant growth in early stages has been reported by the application of digestates, suggesting dilutions below 5% in these periods (Medina et al., 2015), probably explaining the differences in plant biomass as regards Fert treatments. Consequently, the use of these products as stimulants in the initial stages of plant growth and development may be counterproductive, thus higher dilutions are needed. Another important factor to be considered is the substrate used. Previous studies demonstrated that compost combined with digestate is the best treatment for plant growth, even at a similar level or above commercial substrate and chemical fertilizers 14. All this suggests complex interactions among biostimulants, substrates, and plants, being important for phenologic stage, concentration, and frequency of product application.
Chlorophyll content is highly correlated to N content in leaves and may be used as a nutritional indicator 23. All digestates significantly increased this parameter, suggesting the nutritional beneficial effect, mainly N intake in lettuce and tomato plants. Although bioproduct composition presents macro and micronutrients, they also contribute with microorganisms that promote plant growth 18 by mechanisms like hormone production, nutrient solubilization, and N2 fixation. This may explain the increase in plant biomass, despite the lower nutrient content with respect to inorganic fertilizer, indicating that in a bioproduct, microbial content may be more important than nutrient concentration.
Stress indicators Fv/Fm and PIabs, suggested no bioslurries negative effect on plant growth. In lettuce, those plants treated with inorganic fertilizer presented the lowest values, suggesting higher photosystem II damage with respect to digestates and water control. According to the manufacturer (Hansatech Instruments Ltd.), Fv/Fm near 0.85 indicates healthy tissues. Therefore, tomato showed greater damage than lettuce since the values were lower, being control the most affected and suggesting nutritional limitations. PIabs represents plant capacity to respond to stress, being more sensitive than Fv/Fm for stress determinations 2. Despite the high variability between lettuce and tomato, PIabs was significantly increased by Fert, possibly given to higher nutrient intake. Despite the lack of significance, all bioslurries increased these parameters, suggesting an improvement in plant coping ability under biotic and abiotic stress.
Our results demonstrated that digestates can be used as biostimulants for plant growth, with different properties depending on the source and brewing method. Dilutions are needed, due to the toxicity of the pure product, especially for seedling production, which may require even lower concentrations. Further studies are needed to determine dilutions, appropriate moment and frequency of application, and the possibility of combining the different digestates for optimal plant growth, allowing for reduced synthetic products, with lower negative impacts and safer production strategies.
Conclusion
Digestates are valuable by-products, rich in nutrients and microorganisms for high-quality plant production. Each product presented different characteristics and effects on plant biomass, suggesting complex interactions, thus consequent possible complementation in their use. All digestates stimulated plant growth. Bfao showed the highest effect on tomatoes and lettuce biomass followed by Blac and Biog, constituting an adequate alternative for a cheaper, safer and low-impact strategy for crop growth. The biostimulants presented high nutrient content and no phytotoxic effects at concentrations of 10%, being an excellent strategy to treat organic residues while high-quality by-products are obtained. Further studies are needed to determine optimal brewing conditions, dilutions, raw materials and application techniques for producers. Moreover, Liquid biofertilizers should be used and evaluated, not only as an isolated practice but also within a set of sustainable crop management strategies.














