Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.115 no.1 Cap. Fed. maio 2023
http://dx.doi.org/10.25132/raac.v115.n1.1702
Articles
Laparoscopic left pancreatectomy: is systematization of the technique useful?
Introduction
Laparoscopic pancreatic resections should only be performed by surgeons specialized in pancreatic surgery and in high-complexity laparoscopic procedures. Although left pancreatectomy is a procedure technically simpler than pancreaticoduodenectomy, it is associated with 30-50% of complications and with mortality rate of 1-4%1. Therefore, this procedure should not be underestimated.
Left pancreatectomy can be performed with or without splenic preservation. In cases of cancer, the spleen must be resected to achieve proper oncologic resection or as a result of invasion of the splenic hilum. In some patients with benign disease, the spleen can be preserved to avoid postsplenectomy sepsis, a very rare complication with an incidence of 0.9% in patients > 16 years2. There are two techniques for spleen-preserving left pancreatectomy. The Warshaw technique consists of ligation of the splenic vessels, preserving spleen blood flow through the short gastric vessels and the left gastroepiploic artery. The other technique preserves the splenic artery and vein. The use of the Warshaw technique is associated with development of perigastric and submucosal gastric varices in 68% of patients, with risk of intraluminal bleeding3. Another complication is that the remnant spleen function is also impaired in 60% of the cases. This has been demonstrated by the detection of Howell Jolly bodies and the so-called “pitted cells” in peripheral blood3. In the splenic vesselpreserving technique, varices may also develop in the splenogastric circulation4. For these reasons, since the publication of the study3, we have always performed left pancreatectomy and splenectomy either through laparotomy or laparoscopy.
Laparoscopic pancreatectomy and splenectomy require systematization of the surgical steps to reduce the operative time and ensure safe outcomes.
The aim of the present study was to describe the results achieved with a systematized technique for laparoscopic pancreatectomy and splenectomy.
Materials and methods
All the patients undergoing left pancreatectomy between May 2007 and January 2002 were analyzed. All the procedures were performed by the same surgeon and assistants specialized in conventional pancreatic surgery and high-complexity laparoscopic procedures, in both private and public institutions. Data were retrospectively retrieved from a specially designed database and included information about the patient (age, sex, comorbidities, previous surgeries, ASA grade), disease (reason for consultation, time of disease progression, type of disease, weight loss), surgery (type of approach, transfusion requirements, need for transfusions, type of pancreatic section, consistency of the pancreas, operative time), and postoperative course (complications, length of stay in the intensive care unit and general ward, time to resume oral intake, need for enteral/parenteral nutrition, reoperations and histopathology report).
Patients with indication of left pancreatectomy due to neoplasms or pancreatic inflammatory diseases were included in the analysis, while those with distant metastases, ascites or peritoneal carcinomatosis were excluded.
It should be noted that, at the beginning of the series, during the learning curve for laparoscopic resection, patients with small benign lesions distant from the mesenteric vessels were selected for this approach. Currently, we do not perform the laparoscopic approach only in patients with vascular involvement of the celiac trunk or portal vein documented by imaging tests that require a major vascular resection during preoperative planning, in those who require multivisceral resection, or with contraindications for laparoscopy due to comorbidities detected in the preoperative evaluation or in the presence of severe portal hypertension.
Surgical technique
The patient is positioned in the supine position with legs in a straight line. The surgeon stands on the patient’s right side and the assistant(s) on the left side. Four trocars are placed: a 10-mm optical trocar in the umbilical region, a 5-mm in the right hypochondriac region, a 5-mm trocar in the epigastric region and a 10- mm trocar in the left lumbar region. In obese patients an additional 5 or 10-mm trocar can be placed to the left side of the lumbar region trocar.
The first surgical gesture is to open the gastrocolic omentum and section it along the entire greater curvature without preserving the gastroepiploic arcade. The section is performed by cutting all the short vessels until reaching the gastric fundus. In this way, the spleen is “disconnected” from the stomach. Once the greater curvature has been completely released, the splenic artery is dissected and sectioned. By ligating the splenic artery first venous congestion of the spleen during surgery is avoided, facilitating the laparoscopic mobilization of the spleen at the time of splenectomy. The splenic artery can be sectioned at different levels. When the tumor is in the tail of pancreas, the artery can be easily sectioned at the level of the body of the pancreas. But if the tumor is in the body or close to the neck, it is advisable to dissect the celiac trunk to identify the splenic artery and not mistake it for the hepatic artery (Fig. 1). Dissection of the celiac trunk can begin by resecting lymph node 8A. Resection of this node exposes the hepatic artery and it is then possible to continue with the dissection following this artery to the left (in the direction of the celiac trunk). Dissection can be performed with a monopolar scalpel (hook) or with energy devices. Once the celiac trunk has been dissected, the splenic artery can be sectioned just at its origin (Fig. 2). The splenic artery is ligated and the pancreas is dissected out of the retroperitoneum and sectioned. At the level of the neck, the pancreas is thinner and section is safer. A tunnel is created in the neck above the portal vein and a tape is placed as a landmark. The pancreas is sectioned using mechanical stapler. The type of cartridge (blue or green) is selected according to the thickness of the pancreas. The green cartridge is used in thick pancreases and the blue in thin pancreases. Other options for sectioning are monopolar scalpel or energy devices. In the next step, the splenic vein is dissected and sectioned (Fig. 3). Pancreas can also be sectioned using mechanical stapler (white cartridge), Hem-o-lok® clips, intracorporeal ligation and clips.
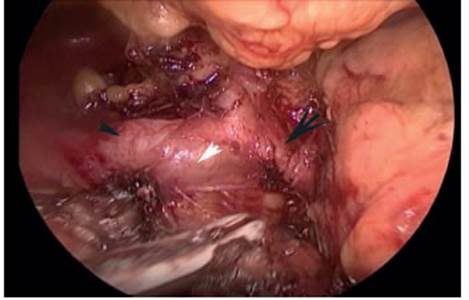
FIGURE 1 Laparoscopic dissection of the celiac trunk to identify the splenic artery at its origin and not mistake it for the hepatic artery Black arrowhead: hepatic artery. White arrowhead: celiac trunk. Black arrow: splenic artery

FIGURE 3 Splenic vein after the pancreatic parenchyma has been sectioned. Black arrowhead: pancreatic parenchyma. White arrowhead: splenic vein
Once all the vascular pedicles have been controlled and the pancreas has been sectioned, left pancreatectomy and splenectomy are performed, if possible, with an energy scalpel. The specimen is placed in a bag and extracted after enlarging the umbilical port site or through a Pfannenstiel-like incision. Two drains are placed, one in the border of the pancreatic section and the other in the splenic bed. At the beginning of the laparoscopic series, we did not perform this systematization of the surgical steps. Because the access to the splenic vein is easier initially, in the first cases, during the learning curve we sectioned the splenic vein first, leaving the section of the splenic artery after sectioning the pancreas. We started using the systematized technique in the case number 25.
Postoperative care
All the patients were admitted to the intensive care unit in the immediate postoperative period. At the beginning of the series, a nasogastric tube was placed in all the patients. Nowadays, nasogastric tubes are not routinely placed. Oral feeding is initiated on postoperative day 2. Early mobilization is attempted as soon as the patient leaves the intensive care unit.
Morbidity and mortality
The definition proposed by the International Study Group of Pancreatic Surgery (ISGPS) was used for the diagnosis of pancreatic fistula, delayed gastric emptying and bleeding5. Complications were categorized using the Clavien-Dindo classification. Drains were removed in the absence of fluid output or biochemical fistula. The patients were discharged when they tolerated an oral diet, were able to walk independently and had no clinical signs of infection.
Statistical analysis
The operative time at the beginning of the laparoscopic series (group 1) was compared with the operative time after case 30 (group 2) once the learning curve had been overcome. Comparisons were made using the Student’s t test for independent samples. A p value < 0.05 was considered statistically significant. The statistical analysis was performed using a Microsoft Excel 16.18® spreadsheet.
Results
During the period described, 160 patients met the inclusion criteria for left pancreatectomy. Five patients were excluded: 3 with liver metastases and 2 with ascites. A total of 155 patients were analyzed, 90 underwent laparoscopy and 65 underwent conventional surgery. Demographic data and tumor size are described in Table 1.
Table 2 shows the intraoperative variables.
The operative time in the laparoscopic approach decreased from case 30 onwards, and this difference was statistically significant (group 1, 181.33 ± 40.23 minutes versus group 2, 157.42 ± 25.22 minutes; p = 0.00489). Table 3 shows incidence of pancreatic fistula and bleeding, length of hospital stay and mortality.
Twenty-three patients required repeat surgery (Clavien-Dindo grade 3). The reasons are described in Table 4.
Conversion rate was 13.3% (12 patients). Three patients were converted for bleeding during spleen mobilization and bleeding from the splenic vein. It should be noted that in these patients, the splenic vein was ligated before ligating the splenic artery, which generated more venous congestion of the spleen. Other conversion was due to tumor invasion of the gastric antrum. The rest of the conversions were due to adhesions in 2 patients, bleeding of the splenic artery in another patient, anatomic factors in 3 patients, and intense inflammation due to pancreatitis before surgery in another 2 patients.
Direct conventional approach was performed in 17 patients, once the learning curve for laparoscopic resection had been overcome. The indications for laparotomy were:
- Contact with or proximity to the celiac trunk (9 patients)
- Large aneurysm of the splenic artery (1 patient)
- Invasion of adjacent organs (4 patients)
- Simultaneous resection of a single liver metastasis of neuroendocrine tumor (1 patient).
- Simultaneous cystogastrostomy and left pancreatectomy for intraductal papillomucinous neoplasm type 3.
Mortality was 1.11% and occurred in a female patient who underwent left pancreatectomy, splenectomy and antrectomy due to gastrinoma in the body of the pancreas, gastric antrum, and 2 liver lesions. Resection was indicated because the patient was refractory to medical treatment and had presented 2 previous episodes of gastrointestinal bleeding. The patient developed a pancreatic fistula type C and required reoperation on postoperative day 6 due to intra-abdominal bleeding with hemodynamic instability. The patient died due to multiple organ failure on day 45. She was the only patient operated on via laparoscopy who underwent resection of another organ besides the pancreas and the spleen.
Table 5 details the histology of the tumors resected of 90 patients undergoing laparoscopic surgery.
Discussion
Minimally invasive pancreatic resections have gained increasing acceptance in our environment, particularly in case of laparoscopic left pancreatectomy. This procedure is performed by most surgeons specialized in pancreatic surgery, since it does not involve a reconstructive stage and is technically less demanding than laparoscopic cephalic pancreaticoduodenectomy. Several publications in the international literature show the advantages of laparoscopic left pancreatectomy compared with the conventional approach. However, most of these studies are not randomized and compare heterogeneous samples, managing smaller tumors by laparoscopy5-9. The latest prospective, randomized, double-blind study (LEOPARD)1 conducted by the Dutch group reveals clear advantages of the laparoscopic approach. Less intraoperative blood loss and the reduction in length of hospital stay were the 2 variables statistically significant observed in favor of the minimally invasive approach. The world literature supports the concept1 that laparoscopic left pancreatectomy is the best option for the patient whenever the procedure is feasible.
Laparoscopic resection is a widely accepted technique for ductal adenocarcinoma of the pancreas. Several studies show that the oncologic outcomes are similar to those of conventional left pancreatectomy5-9. The DIPLOMA study, published by the Dutch group, shows that there is no difference in the survival of patients operated on via the conventional and laparoscopic approaches. There were no differences in R0 resections, and the number of resected nodes was slightly lower in the minimally invasive surgery group, although this difference was not significant10.
Using the laparotomic approach, it is feasible to begin with the vascular axis or with splenectomy and finish the procedure with ligation of the splenic artery. If the laparoscopic approach is performed, systematization of the technique is recommended firstly, and control the vascular axis at the beginning of the procedure. The aim of systematizing the technique described in the “Materials and methods” section is to reduce the operative time, provide a safer resection and reduce the conversion rate by avoiding venous congestion.
Once the learning curve has been overcome, the operative time is reduced. Performing always the same technique is another factor that helps to systematize the surgical steps, and this is very important to reduce the operative time. In the series presented, there was a significant decrease in the operative time with this type of technique since surgery number 30. However, it is difficult to establish the number of surgeries necessary to complete the learning curve. We had performed 25 laparoscopic left pancreatectomies before using this technique, and perhaps the reduction in the operative time is not exclusively due to the systematization of the surgical steps but may have contributed.
Another advantage of the technique described is that resection is safe. Dissection of the celiac trunk allows identification of all the vascular structures and thus avoids accidental section of the hepatic artery. Dokmak11 states that when the pancreatic neck is dissected, the artery that lies just above the neck is the hepatic artery and not the splenic artery. The hepatic artery may be accidentally sectioned due to insufficient dissection, particularly in laparoscopic surgery. This technique might also reduce the conversion rate. In the technique described, we recommend ligation of the splenic artery in first place and then of the splenic vein to avoid venous congestion of the spleen which impairs mobilization of the spleen during splenectomy if the vein is ligated firstly. In our experience, 3 patients underwent conversion due to bleeding from the splenic vein during splenectomy; in these patients, the splenic vein had been ligated firstly. No conversions occurred due to venous bleeding with the technique described. Anyway, the sample is not large enough to make statistical comparisons.
Some factors may affect the laparoscopic approach. Tumor size, tumor location close to vessels, local invasion, presence of segmental portal hypertension and body mass index are relative factors that may be decisive for choosing which approach use. Undoubtedly, the experience of the surgeon in charge is the main factor to indicate the laparoscopic approach. Therefore, the factors previously mentioned take a back seat if the surgeon is highly experienced in high-complexity laparoscopic surgery. In our group, the indication for conventional surgery is currently restricted to cases in which the tumor is close to the celiac trunk (Fig. 4) or in patients requiring multivisceral resection.

FIGURE 4 Computed tomography scan showing a tumor close to the celiac trunk and intraoperative photograph of the tumor in contact with the celiac trunk. Black arrowhead: tumor. White arrowhead: splenic vein emerging from the celiac trunk
At the beginning of the experience, the number of patients approached by laparoscopy was significantly lower than that of those undergoing the conventional approach. Nowadays, this number has been reversed and most patients are managed through laparoscopy. The systematization of the technique made it possible to perform more complex procedures in a safer fashion.
Referencias bibliográficas /References
1. Rooij T, van Hilst J, van Santvoort H, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD) A Multicenter Patientblinded Randomized Controlled Trial. Ann Surg. 2019;269(1):1-9. [ Links ]
2. Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks Br J Surg. 1991;78(9):1031-8. [ Links ]
3. Kohan G, Ocampo G, Zandalazini H, et al. Changes in gastrosplenic circulation and splenic function after distal pancreatectomy with spleen preservation and splenic vessel excision. J Gastronintest. 2013;17(10):1739-43. [ Links ]
4. Yoon Y, Lee K, Han H, et al. Patency of splenic vessels after laparoscopic spleen and splenic vessel-preserving distal pancreatectpmy. Br J Surg. 2009; 96:633-40. [ Links ]
5. Balduzzi A, van Hilst J, Korrel M, et al. Laparoscopic versus open extended radical left pancreatectomy for pancreatic ductal adenocarcinoma: an international propensity score matched study. Surg Endosc. 2021;35(12):6949-59. [ Links ]
6. Sahakyan M, Kleive D, Kazaryan A, et al. Extended laparoscopic distal pancreatectomy for adenocarcinoma in the body and tail of the pancreas: a single-center experience. Langenbecks Arch Surg. 2018;403(8):941-8. [ Links ]
7. Ricci C, Casadei R, Taffurelli G, et al. Laparoscopic versus open distal pancreatectomy for ductal adenocarcinoma: a systematic review and meta-analysis. J Gastrointest Surg 2015;19(4):770-81. [ Links ]
8. Christen J, Kendrick M, Nagorney D, et al. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the páncreas. J Gastrointest Surg. 2005;9(7):922-7. [ Links ]
9. Mazza O, Sahovaler A, Fernández D y col. Pancreatectomías distales laparoscópicas: nuestra experiencia. Rev Argent Cirug. 2015;107(2):51-6. [ Links ]
10. Van Hilst J, Rooij T, Klompmaker S, et al. Minimally invasive versus open distal pancreatectomy for ductal adenocacinoma (DIPLOMA). Ann Surg. 2019;269(1):10-7. [ Links ]
11. Dokmak S, Aussilhou B, Fteriche F, et al. Laparoscopic distal pancreatectomy: Surgical technique. J Visc Surg. 2019;156:139- 45. [ Links ]
Received: April 10, 2022; Accepted: July 25, 2022











 texto em
texto em 








