Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista agronómica del noroeste argentino
versión impresa ISSN 0080-2069versión On-line ISSN 2314-369X
Rev. agron. noroeste arg. vol.36 no.1 San Miguel de Tucumán jul. 2016
SHORT COMMUNICATION
Solubilization of different sources of insoluble phosphate by Gluconacetobacter diazotrophicus
Solubilización de diferentes fuentes de fosfatos insolubles por Gluconacetobacter diazotrophicus
P. Delaporte-Quintana1; M. Grillo-Puertas2; N.C. Lovaisa1; V.A. Rapisarda2; K.R. Teixeira3; R.O. Pedraza1*
1Facultad de Agronomía y Zootecnia, Universidad Nacional de Tucumán. Av. Kirchner 1900, (4000), San Miguel de Tucumán, Tucumán, Argentina. *Email: raulemilioreal@gmail.com
2Instituto Superior de Investigaciones Biológicas (INSIBIO), CONICET-UNT, and Instituto de Química Biológica “Dr. Bernabé Bloj”, Facultad de Bioquímica, Química y Farmacia, UNT, Chacabuco 46, (T4000ILI), San Miguel de Tucumán, Tucumán. Argentina.
3Laboratório de Genética e Bioquímica, EMBRAPA Agrobiologia. BR465 Km 07, CEP 23891-000, Seropédica, Rio de Janeiro, Brazil.
Abstract
The aim of this work was to assess the phosphorus-solubilizing capacity of Gluconacetobacter diazotrophicus under different sources of insoluble phosphate (Pi), using the wild-type strain PAL5 and its Tn5-derivative mutants (K416, 16D10 and 16G6) defectives in organic acids production. Pi solubilization capacity was assessed in NBRIP solid minimal medium, supplemented with different Pi-insoluble sources, by the determination of the solubilization index (SI). The PAL5 strain solubilized all the Pi-sources used herein, with different degree of SI, confirming the Pi-solubilizer status for G. diazotrophicus. K416 and 16G6 mutants were able to solubilize some of the Pi sources tested although with less ability, compared to PAL5. 16D10 was completely unable to solubilize any Pi source tested. Further studies are necessary to perform in order to investigate the participation of G. diazotrophicus and its derivative mutants in the bacterial Pi-metabolism and in association to plants as growth promoters.
Keywords: Phosphate solubilization; Gluconic acid; Gluconacetobacter diazotrophicus PAL5.
Resumen
El objetivo de este trabajo fue evaluar la capacidad solubilizadora de diferentes fuentes fosfatadas insolubles por G. diazotrophicus, considerando la cepa tipo PAL5 y sus mutantes por inserción de Tn5 (K416, 16D10 y 16G6) deficientes en la producción de ácidos orgánicos. La capacidad solubilizadora de fosfatos se evaluó de manera independiente en medio mínimo NBRIP sólido, suplementado con fosfato dicálcico, fosfato tricálcico, hidroxiapatita, escorias Thomas, y fosfato férrico, mediante el cálculo del índice de solubilización (IS). La cepa salvaje PAL5 solubilizó todas las fuentes fosfatadas utilizadas con diferente grado de IS. Las cepas mutantes K416 y 16G6 solubilizaron solo algunas fuentes fosfatadas, con menor capacidad que la cepa PAL5. La cepa 16D10 no solubilizó ninguna de las fuentes fosfatadas evaluadas. Es necesario llevar a cabo otros estudios para investigar la participación de G. diazotrophicus y sus mutantes, en el metabolismo bacteriano del Pi y en asociación con plantas como bacteria promotora del crecimiento.
Palabras clave: Solubilización de fosfatos; Ácido glucónico; Gluconacetobacter diazotrophicus PAL5.
Received 04/13/16; Accepted 06/03/16.
The authors declare to have no conflict of interests.
Gluconacetobacter diazotrophicus (former Acetobacter diazotrophicus) was first isolated from roots, stems, and leaves of sugarcane in Brazil, and then from other agricultural crops such as sugar beet, rice, pineapple, coffee and carrot, among others. Due to its capacity to improve plant yields through different mechanisms of action (e.g., biological nitrogen fixation, auxin production, mineral solubilization, biocontrol activity) it is considered a plant growth-promoting bacterium (Reis and Teixeira, 2015, and its references).
It was reported the capacity of G. diazotrophicus to solubilize insoluble inorganic phosphate compounds (Pi) as a consequence of gluconic acid production, which allows it to be considered within the Pi-solubilizing bacteria (PSB) group (Maheshkumar et al., 1999). In most cases studied, tricalcium phosphate (TCP) or another single source of phosphate were used to report this bacterial characteristic. However, Bashan et al. (2013) have reported that TCP is inappropriate as a universal selection factor for testing PSB that enhance plant-growth, suggesting to use a combination of different P-compounds together or in tandem, as well as testing for the production of organic acids. In a previous work from a library of random transposon insertion three mutants of G. diazotrophicus PAL5 were selected under specific conditions related with the glucose oxidation to gluconic acid production: K416, related with the key enzyme for glucose oxidation to gluconic acid (pyrroloquinoline quinone-glucose dehydrogenase); 16G6, related to a subunity of NADH-quinone oxidoreductase; and 16D10, a putative transmembrane protein with unknown function (da Silva et al., 2009; Vidal et al., 2009). According to this, the aim of this work was to test the Pi-solubilizing capacity of wild-type G. diazotrophicus PAL5 (ATTC 49037)and of K416, 16D10 and 16G6 mutants.
For the P-solubilization assay, cells of each strain were first grown in M1 liquid medium (Gupta et al., 1996), containing 100 µg mL-1 Kanamycin (except for the wild strain PAL5). After 24-48 h of incubation at 30 ºC and 200 rpm, cells were collected by centrifugation (10,000 rpm; 10 min). The pellet was washed twice in sterile distilled water (10,000 rpm; 5 min), resuspended in sterile distilled water and adjusted to OD560 nm = 0.3. Aliquots of 10 µl were placed by triplicate in NBRIP solid medium (Nautiyal, 1999), supplemented with 5 g L-1 of dicalcium phosphate [CaHPO4], tricalcium phosphate [Ca3(PO4)2], hydroxyapatite [Ca5(PO4)3OH], basis slag (containing a mixture of minerals besides phosphorous), or ferric phosphate (FePO4). After incubation at 30ºC during 120 h, the solubilization index (SI) was determined as previously described by Edi-Premono et al. (1996). A positive reaction was determined as the presence of clear halos around the colonies. Results were subjected to analysis of variance and Tukey test (α = 0.05).
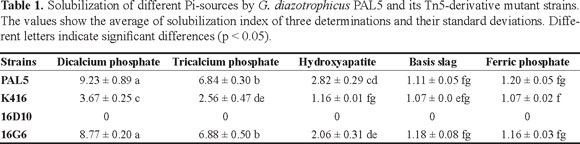
As result, the wild-type strain PAL5 solubilized all the Pi sources used herein, although in different degree, according to their SI (Table 1; Figure 1). This reconfirms the Pi solubilizer status of G. diazotrophicus, demonstrated not only with less insoluble phosphates (e.g., dicalcium and tricalcium phosphate), but also with more insoluble sources such as hydroxyapatite, basis slag, and Fe-(III) phosphate. About the mutant strains, K416, 16G6 were able to solubilize all the Pi sources tested. However, K416 strain showed a reduced solubilizing capacity as compared to PAL5. The 16D10 strain was completely unable to solubilize any Pi sources. Interestingly, although the Tn5 insertion in its genome, the strain 16G6 has showed a similar solubilizing performance than PAL5 (Table 1).
In a previous work, HPLC analysis showed that all strains produced gluconic acid in different amounts (da Silva et al., 2009), being higher for PAL5 in the third day of assessment; almost the same for 16G6 until the second day, and lower for K416 and 16D10 during the 3 days, as compared with PAL5. These results correlate with those for halo formation on NBRIP medium, except for the strain 16D10, that even the low amount of gluconic acid produced, halo formation was not observed. Further studies are necessary to perform in order to investigate the participation of G. diazotrophicus and its derivative mutants in the bacterial P-metabolism and in association to plants as growth promoters.
Acknowledgements
This work was supported by grants from EMBRAPA MP3 03.06.09.013.00, Pronex-FAPERJ Process No. E.26/171.523/2006, INCT-FBN/CNPq and PIUNT A526. PDQ, MGP, and NCL are fellows of CONICET. Part of the results were presented in the III Argentine Congress of Agricultural and Environmental Microbiology (CAMAYA); November 2015, Buenos Aires, Argentina. (http://www.camaya2015.aam.org.ar/descarga/Libro-resumenes-IIICAMAYA2015.pdf).
References
1. Bashan Y., Kamnev A.A., de-Bashan L.E. (2013). Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biology and Fertility of Soils 49: 465-479. [ Links ]
2. da Silva R.J., De Oliveira Godoy R.L., Santos da Rosa J., Tavares da Silva Souza V., Stephan M.P., Dos Santos Teixeira K.R. (2009). Produção de ácidos orgánicos associada à fixação de nitrogênio em mutantes de Gluconacetobacter diazotrophicus. Seropédica, RJ, Brasil. Embrapa Agrobiologia. Boletim de Pesquisa e Desenvolvimento 53. [ Links ]
3. Edi–Premono M., Moawad M.A., Vleck P.L.G. (1996). Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indonesian Journal of Crop Science 11: 13-23.
4. Gupta A., Felder M., Verma V., Cullum J., Qazi G.N. (1999). A mutant of Gluconobacter oxydans defficient in gluconic acid dehydrogenase. FEMS Microbiology Letters 179: 501-506. [ Links ]
5. Maheshkumar K.S., Krishnaraj P.U., Alagawadi A.R. (1999). Mineral phosphate solubilizing activity of Acetobacter diazotrophicus: a bacterium associated with sugar cane. Current Science 76: 874-875. [ Links ]
6. Nautiyal C.S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters 170: 265-270. [ Links ]
7. Reis V.M., Teixeira K.R.S. (2015). Nitrogen fixing bacteria in the family Acetobacteraceae and their role in agriculture. Journal of Basic Microbiology 54: 1-19. [ Links ]
8. Vidal M.S., Soares C.P., Fernandes S.M.A., Silva R.J., Teixeira K.R.S. (2009). Seleção de mutantes de Gluconacetobacter diazotrophicus defectivos na síntese de ácidos orgânicos. Congreso Brasileiro de Microbiologia. 8-12 November, Porto de Galinhas, Brazil. CD-ROM presentation 2263-1. [ Links ]