Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de nefrologia, dialisis y trasplante
versión On-line ISSN 2346-8548
Rev. nefrol. dial. traspl. vol.39 no.1 Ciudad Autónoma de Buenos Aires mar. 2019
ORIGINAL ARTICLE
SYMPATHETIC ACTIVITY IS NEGATIVELY ASSOCIATED TO UREMIC STATE AND HEMODYNAMIC INSTABILITY DURING HEMODIALYSIS SESSIONS
LA ACTIVIDAD SIMPÍTICA ESTÍ NEGATIVAMENTE ASOCIADA AL ESTADO URÉMICO Y A LA INESTABILIDAD HEMODINÍMICA DURANTE LAS SESIONES DE HEMODIÍLISIS
Tiago Ferraz Mascarenhas1, Murilo Carneiro Macedo1,3, Débora Martins da Silva1,3, Lucas Brasileiro Lemos1,2, Fernando Costa Vieira1,2, Jonas R.D. Silva3,4, David Lomanto Couto3,4, Alinne Alves Oliveira3,4, Rafael Pereira13,4
1) Medicina, Universidade Estadual do Sudoeste da Bahia (UESB), Jequié, Bahia, Brasil
2) Centro de Doenͧas Renais de Jequié (CDRJ), Jequié, Bahia, Brasil
3) Grupo de Pesquisa em Fisiologia Neuromuscular, Departamento de Ciͪncias Biológicas, Universidade Estadual do Sudoeste da Bahia (UESB), Jequié, Bahia, Brasil
4) Departamento de Ciencias Biológicas, Universidade Estadual do Sudoeste da Bahia (UESB), Jequié, Bahia, Brasil
5)Centro Integrado de Pesquisas em Fisiologia, Departamento de Ciͪncias Biológicas, Universidade Estadual do Sudoeste da Bahia (UESB), Jequié, Bahia, Brasil
e-mail: carlosm.callegari@gmail.com
Recibido: 6 de julio de 2018
Aceptación final: 16 de septiembre de 2018
ABSTRACT
Introduction: Hemodynamic instabilities, characterized by oscillations of blood pressure, are common during hemodialysis sessions (HD), culminating in intradialytic hypotension owing to volume withdrawal from the cardiovascular system. The ability to carry out immediate adjusts in cardiovascular system, mainly mediated by the autonomic nervous system, is essential to the maintenance of hemodynamic stability during HD. Objective: This study aimed to investigate the relationship between the sympathetic activity, and the hemodynamic stability from chronic kidney disease (CKD) patients during the HD, as well as the relationship between sympathetic activity and the uremic state. Methods: Fourteen CKD patients (8 women and 6 men) with no history of recurrent intradialytic hypotension (ID) episodes had the successive RR intervals recorded during HD. Blood pressure measurements were recorded at regular intervals of 30 minutes along 4 hours of each session. Hemodynamic stability was established by the standard deviation (SD), coefficient of variation (CV) and the Delta (difference between the highest and the lowest measure) of systolic (SBP), diastolic (DBP), and mean (MBP) blood pressures, as well as the pulse pressure (PP) from the 8 recordings obtained during each session. As a measure of autonomic heart control, the low-transformed low frequency (lnLFnu) spectral band was used. The uremic state was established by the mean of uremia from the last 12 months. Pearson's correlation was used to analyze the correlation between the studied variables. Results: The lnLFnu values were negatively associated SD (SBP [r = -0.480; p = 0.010], PP [r = -0.504; p = 0.006] and MBP [r = -0.449; p = 0.017]), CV (SBP [r = -0.390; p = 0.040]) and delta (SBP [r = -0.438; p = 0.020], PP [r = -0.490; p = 0.008] and MBP [r = -0.382; p = 0.045]). lnLFnu was also negatively associated to the uremic state (r = -0.601; p = 0.01). Conclusions: Our results indicate that higher values of the lnLFnu are associated with better hemodynamic stability (i.e., smaller blood pressure oscillations) during HD sessions, in turn, the mean of blood urea concentration in the last 12 months, defined here as the uremic state, was associated with lower values of the lnLFnu during HD sessions.
KEYWORDS: chronic kidney disease; heart rate variability; intradialytic hypotension; blood pressure; sympathetic activity
RESUMEN
Introducción: La inestabilidad hemodinámica, que se caracteriza por las oscilaciones de la presión arterial, es frecuente durante las sesiones de hemodiáilisis (HD) y tiene como resultado la hipotensión intradialítica, causada por una disminución en el volumen sanguíneo del sistema cardiovascular. Es esencial realizar ajustes inmediatos en el sistema cardiovascular, mediados principalmente por el sistema nervioso autónomo, a fin de mantener la estabilidad hemodinámica durante la hemodiálisis. Objetivo: El objetivo de nuestro estudio fue investigar la relación entre la actividad del sistema nervioso simpático y la estabilidad hemodinámica en pacientes con enfermedad renal crónica (ERC) durante las sesiones de hemodiálisis, así como la relación entre la actividad del sistema nervioso simpático y el estado urémico. Material y métodos: Se registraron, durante las sesiones de hemodiálisis, los intervalos RR sucesivos de 14 pacientes con enfermedad renal crónica (8 mujeres y 6 hombres) sin antecedentes de episodios recurrentes de hipotensión intradialítica (HI). Se realizaron registros de la tensión arterial en intervalos regulares de 30 minutos durante 4 horas en cada sesión. La estabilidad hemodinámica se estableció mediante la desviación estándar, el coeficiente de variación (CV) y delta (diferencia entre la medida más alta y la más baja) de la tensión arterial sistólica (TAS), la diastólica (TAD) y la media (TAM), así como la tensión diferencial (TD) a partir de los ocho registros obtenidos durante cada sesión. Se utilizó el análisis espectral de transformaciones logarítmicas de baja frecuencia (LnLFnu, por su sigla en inglés) expresados en unidades normalizadas mediante transformación logarítmica. El estado urémico se determinó a través del promedio de los valores de uremia obtenidos durante los últimos doce meses. Se utilizó el coeficiente de correlación de Pearson para analizar las variables estudiadas. Resultados: Mediante los distintos cálculos, se hallaron las siguientes correlaciones negativas con los valores de lnLFnu : SD (TAS [r = -0,480; p = 0,010]; TD [r = -0,504; p = 0,006] , y TAM [r = -0,449; p = 0,017]); CV (TAS [r = -0,390; p = 0,040]); y delta (TAS [r = -0,438; p = 0,020]; TD [r = -0,490; p = 0,008], y TAM [r = -0,382; p = 0,045]). También se observó una correlación negativa entre lnLFnu y el estado urémico (r = -0,601; p = 0,01). Conclusiones: Nuestros resultados indican que los valores más elevados de LnLFnu se asocian con una mejor estabilidad hemodinámica, es decir, menor oscilación de la tensión arterial, durante las sesiones de hemodiálisis. A su vez, el promedio de concentración de urea en sangre registrado durante los últimos doce meses, al cual definimos como el estado urémico, se relacionó con valores más bajos de LnLFnu durante las sesiones de hemodiálisis.
PALABRAS CLAVE: enfermedad renal crónica; variabilidad de la frecuencia cardíaca; hipotensión intradialítica; presión arterial; actividad simpática
INTRODUCTION
Hemodialysis is an indispensable therapy for patients with renal failure, but it is associated to many adverse episodes, as the intradialytic hypotension (IDH), which is the most frequent adverse complication during hemodialysis sessions.(1-4)
Factors predisposing to hypotension during hemodialysis may be listed as patient-related factors (age, sex, decreased cardiac reserve, autonomic insufficiency, etc.) and dialysis-related factors (rapid decline in plasma osmolality, decrease in peripheral vascular resistance induced by dialysate buffer, high ultrafiltration rates and rapid volume depletion and blood volume shift to dialyzer [200-300 ml], and others).(5-6)
Among patient-related factors, the autonomic insufficiency has been extensively studied.(7-9) While studies investigating the autonomic nervous system (ANS) function in daily activities have shown a reduced cardioprotection, characterized by a reduced vagal activity in patients with chronic kidney disease (CKD), and predisposing these patients to an increased risk of sudden death,(9-15) studies conducted during hemodialysis sessions are less common, but no less important, since the ANS directly influences the hemodynamic control and stability.
Previous studies shown a clear trend to increased sympathetic activity along the hemodialysis session,(3,16) which is justified by the volume withdrawal during dialysis session. The failure in response to the rapid blood volume depletion, by the increase of sympathetic activity, may predispose to hypotension, which is the most frequent complication during hemodialysis sessions.(1-4) This fact gains relevance in the context of patients the maintenance of a high blood urea concentrations, as commonly observed in CKD patients, leads to the uremic autonomic neuropathy, a dysautonomic state similar to observed in diabetics.(17)
The spectral analysis of successive RR intervals have been used to investigate the influence of ANS branch with low and high-frequency spectral bands associated to the predominance of sympathetic and parasympathetic activity, respectively. Previous studies suggested that the predominance of low-frequency band leads to a more stable hemodynamic behavior during hemodialysis sessions.(4,16)
The dialysis-related factors can be easily modified, unlike patient-related factors, which are intrinsic of each patient, requiring in-depth knowledge of patient state to predict patients prone to intradialytic hypotension. Therefore, the knowledge the physiological patient-related variables able to predict the trend to intradialytic hypotension could provide a greater safety and comfort to the HD sessions. In this context, the present study aimed to investigate the relationship between the low-frequency spectral band of the RR interval, an indicator of sympathetic activity, and the hemodynamic stability of chronic kidney disease (CKD) patients during the hemodialysis session, as well as the relationship between this spectral band and the uremic state of these patients.
METHODS
Sample
Fourteen patients, 08 women and 06 men (33 - 9 years old), taken part in the study. They were diagnosed with CKD, undergoing hemodialysis treatment for at least 06 months and have no history of recurrent hypotensive crises during hemodialysis sessions. Diabetes Mellitus (DM) was an exclusion criteria were, since the DM is a major cause of autonomic dysfunction, uncontrolled hypertension (systolic blood pressure - SBP: 200 mmHg and / or diastolic blood pressure - DBP: 120 mmHg) and previous diagnosis of cardiac arrhythmia also were the exclusion criteria. The dialysis time was 73-58 months, the average of blood urea, Urea Reduction Rate (URR) and Kt/V (i.e., fractional clearance of body water for urea) in the last 12 months to the data collection was 122-21 mg/dl, 70-5 % and 1.32-0.13, respectively.
Data collection
The study was conducted at the Kidney Disease Center of Jequie (CDRJ) - Bahia, Brazil from December 2012 to June 2013. The sample consisted from patients with CKD and undergoing hemodialysis treatment. To avoid the influence of circadian rhythm on heart autonomic control, only patients submitted to hemodialysis in the morning were included.
Participants were invited to take part in the study and informed about the study procedures, those who agreed to participate in the study signed an informed consent. The study was approved by the Research Ethics Committee of the State University of Southwest Bahia (protocol#: 09635912.3.0000.0055).
RR interval recording
All patients who met the sample selection criteria and agreed to participate were submitted to the RR intervals recording through a heart rate monitor (Polar® RS800CX, Finland) in two hemodialysis sessions with at least 48 hours apart between them. Heart rate monitors were previously validated for analysis of heart autonomic control18. The hemodialysis sessions lasted for 233-10 and 235-6 minutes, to the first and second session, respectively. The mean weight loss in the sessions (2.61-0.89 and 2.45-0.89 kg to the first and second session, respectively) exhibited an excellent reproducibility between sessions, measured through intraclass correlation coefficient (ICC = 0.94 [95% Confidence Interval: 0.80 ' 0.98]).
The RR interval data was analyzed in the frequency domain using the Fast Fourier transform (FFT) to obtain the following parameters: normalized magnitude from the spectrum of the low frequency components (LFnu) and the high frequency (HFnu). The low and high-frequency power was set as 0.04 to 0.15 and 0.15 to 0.4 Hz, respectively. This procedure was performed with the Kubios HRV analysis software 2.1 (Department of Applied Physics, University of Eastern Finland)(19) and followed the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.(20)
Since the spectral parameter LFnu is reported as a sympathetic activity index,(17) this parameter was used for statistical analysis purposes. In addition, the LFnu values were submitted to a logarithmic transformation (natural logarithm of LFnu [lnLFnu]) as proposed by Fujiwara et al.(21)
Blood pressure recordings and plasma urea concentration
Blood pressure measurements were carried out with automatic blood pressure monitor HEM-OMRON® 742INT. The systolic (SBP) and diastolic (DBP) blood pressures were recorded along each hemodialysis session. During the sessions the recordings were done at the beginning and at each 30 minutes along each hemodialysis session. The pulse pressure (PP) and mean blood pressure (MBP) were calculated from the SBP and DBP measures [PP = SBP-DBP; MBP = DBP + (SBP-DBP / 3)]. As the sessions lasted for approximately 4 hours, a total of eight blood pressure measures were taken. The interval between the penultimate and the last blood pressure measure was smaller than 30 minutes, when the session lasted less than 4 hours.
Hemodynamic stability was established by the mean, the standard deviation (SD), and the coefficient of variation (CV) of SBP, DBP, PP and MBP from the 8 recordings obtained during each session. The delta (i.e., the difference between the highest and the lowest value) of each blood pressure parameter was also used as a hemodynamic parameter. The Figure 1demonstrates the hemodynamic stability and parameters used in this study from a volunteer during HD session.
Blood samples were drawn monthly and urea concentration was quantified by standard laboratorial methods. The average urea concentration of the last 12 months was used as an index of uremic state.
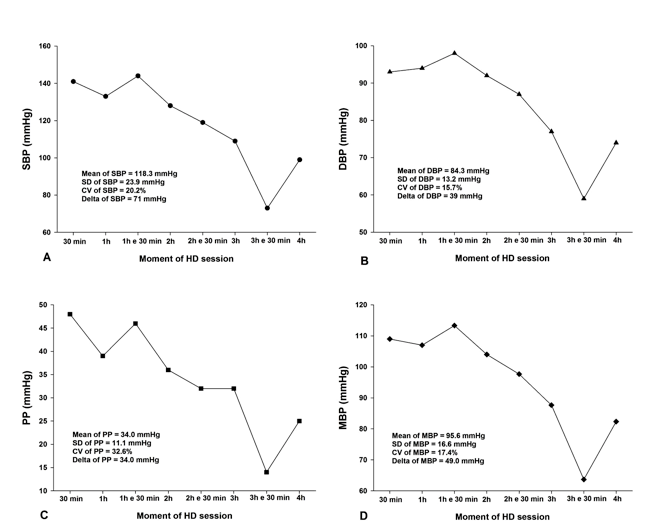
Figure 1. Mean, standard deviation (SD), coefficient of variation (CV), and delta from systolic (SBP), diastolic (DBP), mean blood pressures (MBP), as well as the pulse pressure (PP) of a volunteer during a hemodialysis (HD) session.
Statistical Analysis
Data were presented as mean - standard deviation. Pearson correlation coefficient was used to study relationships between the lnLFnu and hemodynamic stability during hemodialysis session and uremic state. All statistical procedures were performed using SPSS 21.0 (SPSS Inc., IBM, Chicago, IL, USA).
Results
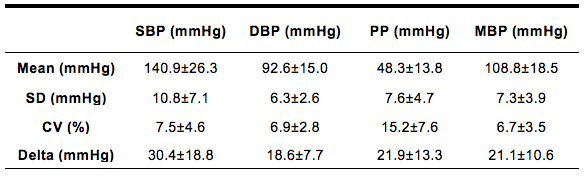
The descriptive characteristics of mean, standard deviation and delta of hemodynamic parameters (pulse pressure, systolic, diastolic and mean blood pressure), used as an index of hemodynamic stability, are presented in Table 1. Â
Table1. Mean, standard deviation (SD), coefficient of variation (CV), and delta from systolic (SBP), diastolic (DBP), mean blood pressures (MBP), as well as the pulse pressure (PP) of a volunteer during a hemodialysis (HD) session.
The Pearson’s correlation analysis between the normalized low-frequency component of RR intervals spectrum (lnLFnu) and hemodynamic stability parameters obtained during hemodialysis sessions revealed that lnLFnu was significantly and negatively correlated to the SD of SBP, PP and MBP, and delta of PP (p<0.05). The correlation coefficients and its respective p values are presented in Table 2.
Table 2. Descriptive characteristics of hemodynamic stability parameters (Standard deviation and Delta of pulse pressure, systolic, diastolic and mean blood pressure) obtained during hemodialysis sessions. --SD = standard deviation; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; MBP = mean blood pressure; CV = Coefficient of Variation.

DISCUSSION
The aim of this study was to investigate the relationship between the lnLFnu, an indicator of sympathetic activity, and the hemodynamic stability of chronic renal disease (CKD) individuals during the hemodialysis session, as well as the relationship between this spectral band and the uremic state of these patients. Our main study finding was that lnLFnu is negatively and significantly correlated to the hemodynamic stability during hemodialysis session. In addition, this spectral parameter, obtained during hemodialysis sessions, is negatively and significantly correlated to uremic state in CKD patients.
The lnLFnu is widely attributed to the sympathetic activity over the cardiovascular system,(17,22) Our results indicated that a lower contribution of LF band leads to a poor hemodynamic stability during the hemodialysis session, which was assessed by the SD, CV and delta of systolic blood pressure, but also for SD and delta of mean and pulse pressures. In fact, the volume withdrawn subsequent to the hemodialysis procedure leads to a reducing trend in blood pressure, and a sympathetic activity may compensate this trend by inducing vasoconstriction, venoconstriction, increasing heart rate and ventricular contraction8. Then, it is expected that, during a hemodialysis session, a highest sympathetic activity may avoid the blood pressure oscillations and drastic falls.
It is noteworthy that spectral analysis of RR intervals is not sufficient to describe all physiological events,(23-24) especially in conditions where external stimuli (such as pain, drug use, menstruation, body position, sleep, among others) happen simultaneously and may have some influence on the spectral parameters. However, the reliability of the spectral parameters as indicators of autonomic nervous system activity on the heart is well established in the scientific literature.(25-26)
Autonomic impairment is common in patients with CKD and a major contributor to this event is the uremic state 27. It is reported that the uremic state in CKD patients is associated to diffuse myocardial fibrosis, coronary artery calcification, left ventricular hypertrophy, endothelial dysfunction(28) and autonomic dysfunction.(8) Such factors would increase the risk of an ejection fraction-independent ventricular arrhythmia(29) and, consequently, may predispose these patients to greater hemodynamic instability.
Other studies, such as Galetta et al.,(7) have shown that the variability of RR intervals is lower in chronic uremic patients, and it is proposed that there is a decrease in both sympathetic and parasympathetic response. In fact, the maintenance of a chronic uremic state leads to uremic autonomic neuropathy, a dysautonomia similar to that observed in diabetic individuals, with a reduction in the parasympathetic cardioprotective activity, followed by a reduction of the sympathetic activity.(17,30) Our results corroborate this statement, since we observed a negative correlation between the uremic state and the spectral band lnLFnu, indicating that the maintenance of high values ''''of blood urea for long time periods can deteriorate the sympathetic action on the cardiovascular system during HD sessions, making these individuals prone episodes of intradialytic hypotension.
Considering that our study included only patients with CKD without recurrent history of intradialytic hypotensive episodes, further studies should investigate the relationship between sympathetic activity and hemodynamic stability, as well as the uremic state from CKD patients with recurrent history of intradialytic hypotension, in order to compare with the results presented here.
In conclusion, our results indicate that higher values ''''of the lnLFnu are associated with better hemodynamic stability during HD sessions, in turn, the mean of blood urea concentration ''''in the last 12 months were associated with lower values ''''of the lnLFnu during HD sessions. Therefore, the maintenance of blood urea concentration within smaller as possible ranges could contribute to avoid hemodynamic instability during HD sessions.
Conflicto de intereses: Los autores declaran no poseer ningún interés comercial o asociativo que presente un conflicto de intereses con el trabajo presentado.
1) Tislér A, Akócsi K, Borbás B, Fazakas L, Ferenczi S, GͶrͶgh S, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2003;18(12):2601-5. [ Links ]
2) Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212-20. [ Links ]
3) Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW. Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodial Int. 2010;14(1):18-28. [ Links ]
4) Sandberg F, Bailón R, Hernando D, Laguna P, Martínez JP, Solem K, et al. Prediction of hypotension in hemodialysis patients. Physiol Meas. 2014;35(9):1885-98.
5) Henrich WL. Hemodynamic instability during hemodialysis. Kidney Int. 1986;30(4):605-12. [ Links ]
6) Davenport A. What are the causes of the ill effects of chronic hemodialysis? Balancing risks: blood pressure targets, intradialytic hypotension, and ischemic brain injury. Semin Dial. 2014;27(1):13-5. [ Links ]
7) Galetta F, Cupisti A, Franzoni F, Morelli E, Caprioli R, Rindi P, et al. Changes in heart rate variability in chronic uremic patients during ultrafiltration and hemodialysis. Blood Purif. 2001;19(4):395-400. [ Links ]
8) Robinson TG, Carr SJ. Cardiovascular autonomic dysfunction in uremia. Kidney Int. 2002;62(6):1921-32. [ Links ]
9) Tory K, Sͼveges Z, Horváth E, Bokor E, Sallay P, Berta K, et al. Autonomic dysfunction in uremia assessed by heart rate variability. Pediatr Nephrol. 2003;18(11):1167-71. [ Links ]
10) Cashion AK, Holmes SL, Arheart KL, Acchiardo SR, Hathaway DK. Heart rate variability and mortality in patients with end stage renal disease. Nephrol Nurs J. 2005;32(2):173-84. [ Links ]
11) Meier P, Vogt P, Blanc E. Ventricular arrhythmias and sudden cardiac death in end-stage renal disease patients on chronic hemodialysis. Nephron. 2001;87(3):199-214. [ Links ]
12) Foley RN, Herzog CA, Collins AJ. Smoking and cardiovascular outcomes in dialysis patients: the United States Renal Data System Wave 2 study. Kidney Int. 2003;63(4):1462-7. [ Links ]
13) Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, et al. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18(2):318-25. [ Links ]
14) Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63(3):1121-9. [ Links ]
15) Kotanko P. Cause and consequences of sympathetic hyperactivity in chronic kidney disease. Blood Purif. 2006;24(1):95-9. [ Links ]
16) Cavalcanti S, Severi S, Enzmann G. Analysis of oscillatory components of short-term heart rate variability in hemodynamically stable and unstable patients during hemodialysis. Artif Organs. 1998;22(2):98-106. [ Links ]
17) Vita G, Bellinghieri G, Trusso A, Costantino G, Santoro D, Monteleone F, et al. Uremic autonomic neuropathy studied by spectral analysis of heart rate. Kidney Int. 1999;56(1):232-7. [ Links ]
18) Gamelin FX, Berthoin S, Bosquet L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med Sci Sports Exerc. 2006;38(5):887-93. [ Links ]
19) Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210-20. [ Links ]
20) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043-65.
21) Fujiwara Y, Sato Y, Shibata Y, Asakura Y, Nishiwaki K, Komatsu T. A greater decrease in blood pressure after spinal anaesthesia in patients with low entropy of the RR interval. Acta Anaesthesiol Scand. 2007;51(9):1161-5. [ Links ]
22) Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol. 2002;84(1):1-14. [ Links ]
23) Mackey MC, Glass L. Oscillation and chaos in physiological control systems. Science. 1977;197(4300):287-9. [ Links ]
24) Wessel N, Riedl M, Kurths J. Is the normal heart rate "chaotic" due to respiration? Chaos. 2009;19(2):028508. [ Links ]
25) Pinna GD, Maestri R, Torunski A, Danilowicz-Szymanowicz L, Szwoch M, La Rovere MT, et al. Heart rate variability measures: a fresh look at reliability. Clin Sci (Lond). 2007;113(3):131-40. [ Links ]
26) Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37(3):163-72. [ Links ]
27) Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol. 2009;5(10):542-51. [ Links ]
28) Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014;7(7):703-14. [ Links ]
29) Waks JW, Tereshchenko LG, Parekh RS. Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J Electrocardiol. 2016;49(6):848-54. [ Links ]
30) da Silva DM, Macedo MC, Lemos LB, Vieira FC, PirÍ´po US, Andrade HB, et al. Reliability analysis of the heart autonomic control parameters during hemodialysis sessions. Biomed Tech (Berl). 2016;61(6):623-30. [ Links ]














