Introduction
Granulomatosis with polyangiitis (GPA) is considered an antigenic necrotizing granulomatous inflammation affecting mainly small and medium caliber vessels (capillaries, venules, arterioles, arteries, and veins), highly associated with cytoplasmic antineutrophilic antibody (ANCA), involving the upper (UA) and lower (LA) respiratory tract as well as other organs1. In Europe, the prevalence of GPA is 2.3-146 cases per 1 million persons2,3, and it can occur in any racial group, but predominantly in Caucasians. Both sexes are involved, however, women have greater involvement of the respiratory tract. The mean age at diagnosis is 40 years3.
Most patients have the classic triad of upper respiratory tract, pulmonary and renal involvement4. Clinically, in the UA and LA, the disease manifests with nasal crusting and congestion, epistaxis, snoring, cough, hemoptysis, dyspnea, stridor, and wheezing. However, otorhinolaryngologic symptoms may present as the first manifestation of GPA, involving 70 to 100% of cases. Although saddle nose is suggestive of GPA this finding is only found in 3.5 to 7% of cases4. In the lung, inflammation and necrosis involve the arteries, veins, capillaries, bronchi/ bronchioles, interstitium and pleura. Some manifestations of LA may be reported as the onset of the disease or as an isolated finding without involvement of other systems5.
Over the years, it is known that findings of UA were associated with chronic colonization of Staphylococcus aureus, showing that nasal carriage of the bacterial agent is approximately three times higher in patients with GPA compared to healthy individuals, due to dysregulation of the immune system by the disease itself and by frequent nasal lesions6,7. Staphylococcus aureus may have some role in the induction of disease activity7,8. Also, a cascade of pathogenic events has been described that begins with the release of neutrophil DNA and ends with the formation of tissue digestive monocytes/macrophages. Manifestations in head and neck include septal perforations, saddle nose deformities, bony erosions of the orbital and sinus walls, middle ear damage and epiglottitis, and connective tissue destruction that are in association with neutrophil extracellular traps (METs) that function as an inducer of danger associated molecular patterns in monocytes specifically S100A910.
The presence of C-ANCA may have a sensitivity of 90 to 95% and specificity of 90% during disease activity. P-ANCA may also be present in up to 20% of cases11. Relapses are frequently preceded by increased ANCA, and few patients have nonreactive ANCA during flares, so this test cannot be used in isolation for therapeutic decisions11,12.
The most important factor in the diagnosis is obtained by pathological findings showing vasculitis, geographic necrosis, microabscesses, granulomatous inflammation, hemorrhage, or mixed inflammatory process, involving small arteries and veins of several organs12. One study showed that vasculitis and necrotizing granulomatous inflammation were detected in 89% of lung biopsies; granulomas and necrosis in 90%; and these three characteristics together in 91% of the samples13.
The aim of the present study is to describe the manifestations of GPA in the airway mucosa, the ANCA profile, and the pathologic findings in a group of patients with a diagnosis of GPA undergoing bronchoscopy.
Methods
The study was approved by the Research Ethics Committee of the Heart Institute - Hospital das Clínicas of the Faculty of Medicine of the University of São Paulo (processing number 4.328.169).
This was a retrospective, single-center study, in which we evaluated the medical records of patients with a diagnosis of GPA (according to the American College of Rheumatology criteria)14 evaluated at the outpatient pulmonary vasculitis clinic of the Pulmonology Department of the institution. All patients underwent bronchoscopy for airway assessment and collection of material according to the clinical indication (transbronchial/endobronchial biopsy or bronchoalveolar lavage, BAL) in the period 2012 to 2019 (Figure 1).
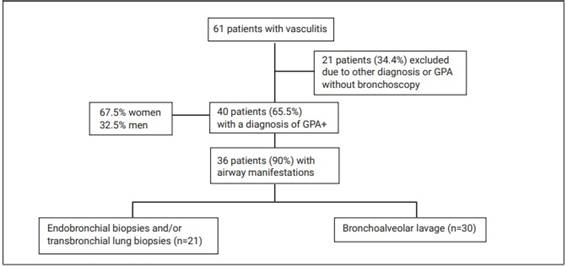
Figure 1: Flow chart of patients included in the study and procedures performed. *ANCA: antineutrophil cytoplasmic antibody. +GPA: granulomatosis with polyangiitis.
Patients who despite being diagnosed with GPA did not undergo bronchoscopy within the specified period, patients with infections at the time of examination, with another type of vasculitis or external cases that had no previous assessment of pulmonary vasculitis service were excluded.
All patients involved in the study underwent flexible bronchoscopy with intravenous sedation with midazolam, fentanyl citrate and propofol, in addition to topical anesthesia of the airway with lidocaine 1%. All patients received supplemental oxygen by nasal catheter and multiparametric monitoring during the examination. The investigation of the endoscopic findings was performed by analyzing the descriptive reports of the examinations and the analysis of the endoscopic images available in the computerized system of the Institution.
In this study, we analyzed the frequency of endoscopic manifestations of GPA, characterized by the presence of granuloma, ulceration, stenosis/synechiae, nodules or masses, polyps, granulosa bronchitis, edema and enanthema, based on the criteria described by Polychronopoulos et al15.
We analyzed the frequency of histopathological findings based on the criteria published by Echeagaray et al, such as: presence of vasculitis, ulcers, necrosis, microabscesses, granulomatous infiltrates, granulomas, multinucleated giant cells16. To facilitate the understanding and analysis of the pathological findings, we chose to group them according to the common characteristics of the descriptions in the reports of the biopsy materials17, presented in Table 1. In addition, we described reactive or non-reactive C-ANCA and P-ANCA, either as the most commonly found pathogens in the BAL.
Table 1: Histopathological findings in patients with granulomatosis with polyangiitis.
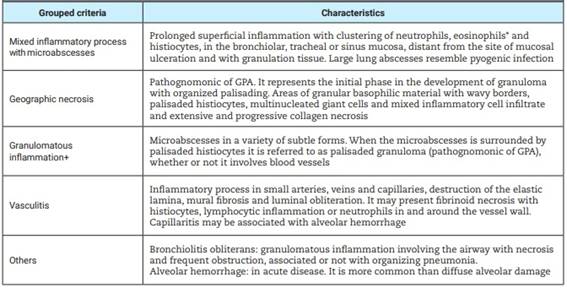
*Small numbers of eosinophils are almost always seen in GPA, but need not be present for diagnosis; multinucleated histiocytes may be present, however, they also occur in other inflammatory processes including infections and sarcoidosis. +They can occupy large regions of parenchyma, including bronchi and alveoli.
The statistical analysis was described and performed using SPSS version 25.0 software. We performed the calculation of simple and relative frequencies of serological, pathological and endoscopic findings.
Results
Forty patients with a diagnosis of GPA were evaluated, 67.5% were female (n=27) and 32.5% male (n=13). The mean age was 46.92±17.61 years (Figure 1).
Of the 40 patients included in the study, 36 (90%) had airway compromise, being more frequent in the LA (86%). The endoscopic findings observed are described in Table 2. The most frequent findings in the UA were: chronic sinusitis (41.7%) and scarring/stenosis (38.9%), respectively. In the LA group bronchi were the most affected, followed by subglottic stenosis and tracheal stenosis. There were patients with more than one involvement of UA, LA or both (Figure 2).
Table 2: Frequency of bronchoscopic findings in 36 patients with granulomatosis with polyangiitis.
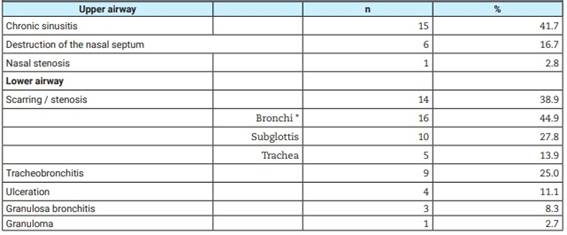
* More than one bronchial segment per patient.

Figure 2: Upper and lower airway involvement in granulomatosis with polyangiitis. A) Inflammatory process of the subglottis mucosa with secretion and discrete fibrotic bands; B) Circumferential cicatricial stenosis of the subglottis with presence of acute inflammatory process and fibrin; C) Complex cicatricial subglottic stenosis; D) Simple cicatricial subglottic stenosis.
If the 36 patients with airway involvement, 63.9% (n=23) had reactive C-ANCA, while 25% (n=9) had reactive P-ANCA, and 8.33% (n=3) had doubly reactive ANCA. In 20% of patients (n=8) ANCA was non-reactive. In the latter group, 87.5% of patients (n=7) presented some degree of airway compromise.
More than half of the patients (61.9%) submitted to biopsies presented mixed inflammatory process with microabscesses. Other findings were geographic necrosis (47,6%), granulomatous inflammatory process (42,9%) and nonspecific alterations (42,9%). Vasculitis with involvement of arteries, veins and capillaries was observed in only 9.52% of cases.
BAL was obtained in 30 patients, and of these, 33.3% had neutrophil counts higher than 5%. Staphylococcus aureus was present in 23.34%, followed by Pseudomonas aeruginosa in 13.3%. Other agents found were Pneumocystis jirovecii, Mycobacterium fortuitum and Serratia marcescens.
Discussion
Granulomatosis with polyangiitis, previously known as Wegener’s disease, is a necrotizing inflammatory vasculitis of small vessels characterized by a classic triad involving the respiratory tract, lungs and kidney, and may manifest in other organs such as skin, gastrointestinal, auditory or central nervous system18.
The literature refers that women and young people (<30 years) present a higher risk of airway involvement18. In our study, more than half of the patients were female (67.5%), with a mean age of 46.92±17.61 years; however, most patients were older than 30 years.
Bronchoscopy continues to be the main diagnostic tool in the evaluation of the severity, location and endoluminal nature of airway lesions in LA (inflammatory or sequelae activity), as well as for the collection of materials (BAL and biopsies). Endoscopic findings include: necrosis of the nasal mucosa, perforation of the nasal septum, masses, edema and ulcers in the tracheobronchial mucosa, as well as tracheobronchomalacia and tracheoesophageal fistula (Figure 3). These manifestations in patients with GPA may be present in 70 to 100% of cases and may be the only manifestation of the disease18. Our study showed that 90% of the patients presented some degree of airway compromise, mainly the LA in more than 80%.

Figure 3: Endoscopic findings in patients with granulomatosis with polyangiitis. A) Inflammatory process of the subglottis mucosa with secretion and discrete fibrotic bands; B) Circumferential cicatricial stenosis of the subglottis with presence of acute inflammatory process and fibrin; C) Complex cicatricial subglottic stenosis; D) Simple cicatricial subglottic stenosis.
Subglottic stenosis is a common cause of stridor in patients with suspected GPA, occurring in up to 50% of them, regardless of disease activity. It is usually recurrent and often refractory to endoscopic dilation therapy19,20. Of the 40 patients included in the study, 28% had subglottic stenosis and 44% had bronchial stenosis (Figure 4). Thus, it is very important that a detailed description of the airway lesions be included in the examination reports in order to be able to monitor them, indicate biopsy collection or treat initial stenosis in a minimally invasive manner with serial endoscopic dilatations, whenever necessary. It is important to emphasize that due to the recurrence of lesions and the possibility of involvement of more than one anatomical site, we prefer to treat with serial endoscopic dilatation. Corticoid injection in the bronchial mucosa can be performed with the objective of delaying the evolution of the cicatricial inflammatory process after dilatation21.
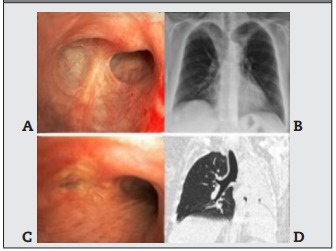
Figure 4: Bronchial stenosis in granulomatosis with polyangiitis. A) Endoscopic view of cicatricial stenosis in the right upper lobe; B) Frontal chest radiograph showing partial atelectasis of the right upper lobe; C) Total stenosis of the left main bronchus with mucosal inflammation; D) Computed tomography view of bronchial stenosis and total atelectasis of the left lung.
The diagnosis of GPA is based on the combination of reactive ANCA serologic manifestations, in particular the cytoplasmic pattern of ANCA (C-ANCA - highly specific), although not essential, associated with histologic evidence described with vasculitis and necrotizing glomerulonephritis or granulomatous inflammation of a relevant organ22.
The most routinely used methods for ANCA testing include: a) indirect immunofluorescence, to detect C-ANCA (cytoplasmic), P-ANCA (perinuclear) and sometimes P-ANCA (for an atypical pattern) and b) ELISA to detect PR3 and/or MPO ANCA21. According to Shirmer et al23, there are differences in the presentation and course of the disease depending on the ANCA phenotype (MPO-ANCA or PR3-ANCA). The main difference found was the high prevalence of disease limited to the airways not threatening other organs or mild systemic disease in reactive MPO-ANCA. In addition, most of the patients in this author’s study were female and had less need for immunosuppression. In our study, it was demonstrated that there was an important percentage of airway involvement and reactive C-ANCA, however, ANCA phenotypes were not analyzed.
On the other hand, histopathological analysis is essential for the diagnosis of GPA. In contrast to open lung biopsy, the result of bronchoscopic biopsy of the tracheobronchial mucosa can provide a diagnosis in only a minority of cases (20% to 25%), as already demonstrated by Devaney et al24. Histopathological findings for the diagnosis of GPA are described as: vasculitis, ulcers, necrosis, microabscesses, granulomatous infiltrates, granulomas, presence of giant cells and fibrotic degeneration.
Tissue necrosis is often the most evident morphologic feature presenting with microabscesses (collection of neutrophils mixed with histiocytes), geographic necrosis or granulomatous inflammation. The first finding takes the form of a neutrophilic microabscess. This micronecrosis constitutes the initial phase in the development of the pathognomonic granuloma of palisading in organization25, as large abscesses in the lung can resemble a pyogenic infection. Vasculitis may present with epithelioid histiocytes, multinucleated giant cells, or both. The presence of lymphocytes in venules may be the cause of hemorrhage and giant cells ingest fragments of elastic fibers from the vessel wall26. In our study, 21 biopsies were analyzed, and the histopathological findings were arranged in 5 groups. Of these findings, the most frequent were: mixed inflammatory process with microabscesses (61.9%), and several tissue samples showed clustering of neutrophils and histiocytes, in addition to geographic necrosis (in 47.61%). Several samples showed extensive and progressive collagen necrosis, however, no sample was described with histiocytes in palisade. In a small number of patients, we had the presence of vasculitis in biopsied tissues.
There are some limitations regarding this study due to the retrospective nature of the study include: difficulty in analyzing examination results, contributing to the exclusion of some patients, failures in medical records and small number of patients arranged in the groups for analysis making statistical correlations difficult.
Furthermore, focusing in greater detail, it was a transversal retrospective study and we did not follow up on all the patients, which limits the understanding of the progression of airway findings. Second, the patients were subject to some treatment variability over time, which limits the understanding of disease progression and eventual outcomes. Finally, ANCA phenotypes weren’t used in our study, which could impact the management of the patients, therefore this omission is clinically relevant, and it might affect the interpretation of the results, as well as the lack of data on follow-up of this patient after the treatment for understanding disease progression.
Conclusion
Airway involvement is common in patients with GPA. Chronic sinusitis, subglottic stenosis and tracheal stenosis were the most frequent findings in our study. This study demonstrated that there was a large percentage of airway involvement associated with reactive C-ANCA, however, we observed non-reactive ANCA in up to 20% of cases. The most frequent histologic findings were polymorphonuclear inflammatory process and geographic necrosis, however, no sample described histiocytes in palisade. The least frequent finding was vasculitis. We suggest studies in the future with the association of these outcomes, ANCA phenotypes, and bronchoscopy of follow-up for understanding the airway compromise in GPA.














