Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Mastozoología neotropical
versión impresa ISSN 0327-9383versión On-line ISSN 1666-0536
Mastozool. neotrop. v.17 n.1 Mendoza ene./jun. 2010
ARTÍCULOS Y NOTAS
Invasive north american Beaver (Castor canadensis): the distribution of mitochondrial variation across the archipelago of Tierra del Fuego
Mariana Fasanella, Sebastián Poljak and Marta S. Lizarralde
Centro Regional de Estudios Genómicos, Universidad Nacional de La Plata, Av. Calchaquí Km 23.5, 4th floor, CP 1888 Florencio Varela, Buenos Aires, Argentina [Corresponding author: Marta S. Lizarralde <mlizarralde@creg.org.ar>].
ABSTRACT: In 1946 twenty-five pairs of Castor canadensis were introduced into the Isla Grande of Tierra del Fuego (Argentina). Thanks both to environmental conditions favorable for colonization and an absence of effective control measures, today the estimated abundance is about 100 000 individuals. We have conducted molecular analysis of beavers from three areas of the Archipelago of Tierra del Fuego, in order to characterize the genetic structure of the invasive population. Our results from AMOVA (Fst= 0.104, p < 0.001) suggest that geographical barriers and large distances could limit gene flow among the populations. In the Tierra del Fuego National Park subpopulation, we found a positive and significant autocorrelation out to 800 m (r = 0.193, p = 0.003) and the greatest haplotype diversity (б= 0.83), which can probably be explained by the control plan used in this area or by natural selection combined with greater habitat diversity. We propose using the information about the demographic spatial dynamics and the spatial genetic structure in this invasive population to design an effective control strategy.
RESUMEN: Castor norteamericano exótico (Castor canadensis): la distribución de la variación mitocondrial en el archipiélago de Tierra del Fuego. En 1946 veinticinco parejas de Castor canadensis fueron introducidas en la Isla Grande de Tierra del Fuego (Argentina). Gracias a las condiciones ambientales favorables para la colonización y a la ausencia de medidas de control efectivas, en la actualidad hay aproximadamente unos 100 000 individuos. Para caracterizar la estructura genética de la población invasora, se llevaron a cabo análisis moleculares en tres subpoblaciones de castores del Archipiélago de Tierra del Fuego. Los resultados del AMOVA (Fst = 0.104, p < 0.001) sugieren la presencia de barreras y de grandes distancias geográficas que podrían limitar el flujo génico entre las poblaciones. Particularmente, en la subpoblación del Parque Nacional de Tierra del Fuego, se encontró una autocorrelación positiva y significativa hasta los 800 m (r = 0.193, p = 0.03) y la mayor diversidad haplotípica (б = 0.83). Esto último, podría deberse al plan de control que se estaba llevando a cabo en esta área o al efecto de la selección natural combinada con una mayor diversidad de hábitat. Finalmente, en este trabajo proponemos utilizar la información sobre dinámica demográfica-espacial y la estructura genético-espacial de la población invasora para diseñar una estrategia de control más efectiva.
Key words. D-loop; Invasive species; Spatial genetic structure; Tierra del Fuego.
Palabras clave. D-loop; Especies invasoras; Estructura genético-espacial; Tierra del Fuego.
INTRODUCTION
Biological invasions, climate change and habitat fragmentation are among the leading threats to the maintenance of global biodiversity (e.g., Sala et al., 2000; Sakai et al., 2001; Travis and Park, 2004; Lizarralde et al., 2008a; Novillo and Ojeda, 2008) and the main cause of species extinctions in island ecosystems (Clout and Veitch, 2002). Such invasions sometimes have considerable impact on local and regional economies (Sala et al., 2000; Vázquez, 2002; Lizarralde et al., 2008a). Studies of colonization processes and exotic population control are thus major topics of concern for conservation biologists and a priority concern for wildlife management (Abdelkrim et al., 2005).
The introduction of exotic wildlife has become a serious problem in Argentina, and Patagonia is particularly vulnerable to invasive species. Most of the introduced exotics have subsequently become established there (Bonino, 1995; Novillo and Ojeda, 2008). In the Archipelago of Tierra del Fuego (ATDF), exotic species represent more than a 67% of the current biota (Poljak et al., 2007). Colonization of this insular area by the North American beaver, Castor canadensis, which has benefited from the absence of both predators and competitors, has had a major ecological impact (Bonino, 1995; Lizarralde and Escobar, 2000; Jacksic et al., 2002). Twenty-five breeding pairs of beavers from Alberta, Canada were introduced into the Claro River in the northeast part of Fagnano Lake, in the Argentinean part of Isla Grande of Tierra del Fuego in 1946, as part of a governmental initiative to promote fur exploitation by introducing exotic furbearers (Godoy, 1963). Since that time, the beaver population has grown exponentially, and has expanded to the rest of the ATDF (Lizarralde, 1993), thanks both to favorable environmental conditions and an absence of effective control measures (Lizarralde and Elisetch, 2002; Lizarralde and Venegas, 2002). By the 1960s, the population had expanded across the Beagle Channel and progressively colonized the Chilean islands of Navarino, Hoste, Picton, Nueva and Lenox. In 1989, the Chilean Navy reported the first beaver colonies on Dawson Island (Anderson et al., 2009). We have previously mentioned a high population density, ranging from 0.2 to 5.85 colony sites per km in the drainage basins, with a radial rate of spread of 2-6 linear km/yr (Lizarralde, 1993; Lizarralde et al., 2004, 2008a). Despite control efforts, the population has increased considerably; today; there are approximately 100 000 individuals, and about 98% of the basins are now occupied by beavers (Coronato et al., 2003; Lizarralde et al., 2008a, 2008b). Recently, initial foci of colonization have been detected on the Brunswick Peninsula in Chile, dating from at least 1994 (Anderson et al., 2006; Skewes et al., 2006; Anderson et al., 2009), confirming that the species was capable of occupying that sector of continental Patagonia. According to Jaksic et al. (2002), beavers have dispersed from Argentina to Chile across the Beagle Channel (ca. 7 km wide) of their own accord. Beaver invasion is fast becoming a pressing problem in Argentina and Chile. Beavers significantly reduce riparian forest canopy up to 30 m away from streams (Anderson et al., 2006) and modify stream morphology and hydrology by building dams, which leads to retention of sediment and organic material in the stream channels (Lizarralde et al., 1996, 2008b).
Although the ecological and economic effects of invasive species have sometimes been evaluated (Jaksic and Fuentes, 1980; Bonino, 1995; Kolar and Lodge, 2001; Jaksic et al., 2002; Vázquez, 2002), our understanding of changes to genetic structure and the evolutionary dynamics of invasive species is still guided more by traditional theory (Kolbe et al., 2004; Frankham, 2005; Lindholm et al., 2005) than by empirical evidence (but see Hampton et al., 2004; Bryan et al., 2005). There has not been much attention devoted to the genetic structure of invading mammals (Abdelkrim et al., 2005; Lecis et al., 2008), but Lizarralde et al. (2008a) provide preliminary information on the genetic structure of beaver populations in the ATDF, demonstrating the fixation of new haplotypes and several nucleotide changes over a short period of time, subsequent to the original establishment of the founder population from North America. Here, we report the results of molecular analysis (using mitochondrial DNA) of beavers from three populations of the ATDF, as a means of characterizing the genetic structure of this invasive population. Mitochondrial DNA can be used as a good phylogenetic marker in the context of mammalian evolution, and in general for vertebrate phylogenetic analysis (Saccone et al., 2000). In particular, mitochondrial DNA markers have been shown to be effective to begin to reveal the present genetic structure and history of populations (Avise, 1998). Mammalian mitochondrial DNA shows strict maternal inheritance, no recombination, and its Control Region (D-loop) is characterized by particularly rapid evolution. These features make it an excellent model for studying the evolutionary relationships among mammals at different divergence levels (Brown et al. 1986, Saccone et al. 2000).
Genetic structure can be studied at both the macro-geographic scale (among populations), using F-statistics (or analogues) and the micro-geographic scale (within populations), by means of spatial autocorrelation analysis. Micro-geographic (fine-scale) spatial analysis has been used to distinguish the genetic structure of several species (Temple et al., 2006; Smouse et al., 2008). With fine-scale spatial genetic analysis, we can study the genetic footprint of restricted dispersion over small spatial scales, inside single subpopulations (Hardy and Vekemans, 1999; Rousset, 2000; Vekemans and Hardy, 2004; Nussey et al., 2005; Valbuena-Carabaña et al., 2007). Such analyses have been particularly useful for plants, which typically have restricted gene flow and genetic structure on a very local scale (Wright, 1943, 1978). Although it seems unlikely that animal species with high dispersal rates would show fine-scale-spatial-genetic-structure, there are exceptions (beavers among them) that might well exhibit very local genetic structure, due to short dispersal distances, strong sociality or territoriality (see also Epperson, 1990; Baker et al., 2000; Peakall et al., 2003; Foerster et al., 2006; Mora et al., 2007; McEachern et al., 2007).
Accordingly, our goal is to analyze the genetic structure and diversity of the beaver population of the ATDF on a small spatial geographic scale.
MATERIALS AND METHODS
Study area
Study sites are located on two islands of the ATDF, Isla Grande (52º 27' 04" S, 69º 32' 08" W and 55º 03' 00" S, 66º 31' 52" W, Argentina) and Isla Dawson (53º 60' 00" S, 70º 38' 00" W, Chile). Isla Grande is the largest of thousands of islands belonging to the archipelago; it shows environmental characteristics that have been previously described in some detail by our research team (Lizarralde, 1993; Martínez Pastur et al., 2006; Lizarralde et al., 2008a). According to Lizarralde (1993), study sites on Isla Grande are characterized by high productivity, with southern beech forest (Nothogafus sp.) and a vast complex basin network, while Isla Dawson has low species richness but high habitat diversity, consisting of a heterogeneous mosaic of ecosystems in close proximity, due to abrupt topography and complex geography (Anderson et al., 2006). Here, we have analyzed populations from three study sites: (1) Fagnano Lake (FLA, Argentina); (2) Tierra Del Fuego National Park (TNP, Argentina); and (3) Isla Dawson (IDC, Chile) (Fig. 1). A total of 111 beaver samples were collected from study sites by park guards, hunters and field collectors (52 from FLA, 38 from TNP and 21 from IDC). The last set was provided by Nicolás Soto (Servicio Agrícola Ganadero de Chile).

Fig. 1. Archipelago of Tierra del Fuego (Argentina-Chile). Numbers indicate the three sampling sites: (1) Fagnano Lake (Argentina, FLA); (2) Tierra del Fuego National Park (Argentina, TNP) and (3) Isla Dawson (Chile, IDC).
Laboratory Protocols
We extracted DNA from fresh tissues (liver, muscle or spleen) and dried skin, using the sodium dodecyl sulfate-proteinase K/phenol-chloroform/RNAse method and concentrated the extracted DNA by ethanol precipitation (Sambroock et al., 1989). We have referenced tissues and other data associated with each specimen directly to each voucher specimen and have stored them, along with field catalog number, in the official collection of the Laboratory of Molecular Ecology (Centro Regional de Estudios Genómicos, Florencio Varela, Argentina).
We amplified the mitochondrial control region (D-loop, 503 bp) with universal primers: Thr-L15926 (5´-CAATTCCCCGGTCTTGTAAACC-3´), located in the neighboring tRNA-pro gene, and DL-H16340 (5´CCTGAAGTAGGAACCAGATG-3´), following Vilà et al. (1999). We performed the amplification of the double-stranded product in 25 μl total reaction volume, with two polymerase chain reaction (PCR) thermal profiles, using Thermus aquaticus DNA-polymerase in a THERMO HYBAID MBS 0.2S. A 25 μl of PCR mixture contained 50-100 ng of DNA, 1.25 U of Taq DNA Polimerase (Promega™ Taq B, GenBiotech), 2.5 μl of 10 x Taq polymerase buffer, with (NH4)SO4, 1.5 mM of MgCl2, 200 μM of each dNTP and 5 μM of each primer. Thermal profiles of D-loop consisted of denaturation for 30 sec at 94ºC, annealing for 30 sec at 55ºC, and extension for 30 sec at 73ºC; we repeated this cycle 40 times.
We purified and sequenced double-stranded PCR products in both directions, using the amplification primers. We sequenced on an ABI Prism Automated 3130 XL (Applied BiosystemsTM) sequencer at the Division of Sequencing Services, Universidad Nacional de Buenos Aires (UBA, Buenos Aires, Argentina). We edited the sequences visually, and managed and aligned them with CHROMAS 2.3 (Technelysium Pty. Ltd. 1998-2004, available from http://www.technelysium.com.au), using the CLUSTAL W software (Thompson et al., 1994). We then optimized the alignments manually.
Statistical Analyses
We calculated divergence among subpopulation (Fst), along with haplotypic (π) and nucleotide (δ) diversity, using ARLEQUIN Version 3.0 software (Weir and Cockerham, 1984; Excoffier et al., 1992, 2005).
In order to investigate the possibility of hierarchical population structure, we conducted an Analysis of Molecular Variance (AMOVA; Excoffier et al., 1992), using ARLEQUIN Version 3.0 (Excoffier et al., 2005). Although Fst estimates the genetic-population structure, providing a sense of the impact of dispersal between discrete subpopulations, it is insensitive to more restricted dispersal within populations. Thus, we have also conducted spatial autocorrelation analysis (SAA), using GenAIEx 6.1 (Peakall and Smouse, 2006), in order to identify the patterns of genetic affinity over shorter distances, a better gauge of local dispersal (Smouse and Peakall, 1999; Peakall et al., 2003; Smouse et al., 2008).
For SAA, we restricted attention solely to the TNP subpopulation, where we have (n = 38) previously sampled animals, each with precise geographic coordinates recorded in the historical register (Lizarralde, 1993; Lizarralde and Elisetch, 2002; Martínez Pastur et al., 2006; Lizarralde et al., 2008a). We defined six distance classes with approximately equal numbers of pairs: 0-0.8 km, 0.8-2 km, 2-3.5 km, 3.5-5 km, 5-8 km, 8-11 km.
RESULTS
Sequence Divergence
We identified seven unique D-loop haplotypes among 111 individuals of C. canadensis, six of which (Hap A-F) have been reported earlier (GenBank Accession numbers: AY787822-27) by Lizarralde et al. (2008a). We have also deposited the sequence of an additional haplotype (H) in GenBank (Accession number: EU476079). After alignment, there were six segregating sites (Table 1), all located at the 3'- end of the amplified fragment.
Table 1 Haplotype definition, haplotype frequency, nucleotide diversity (π, with % standard errors SE) and haplotype diversity (δ, with % standard errors SE) to the control region set of sequences in the analyzed populations. Only polymorphic sites are presented: see GenBank accession numbers AY787822-AY787827 and EU476079 for full sequences. Vertical digits indicate nucleotide position relative to Hap A. "." indicates identity with Hap A. FLA = Fagnano Lake; TNP = Tierra del Fuego National Park; IDC = Isla Dawson.
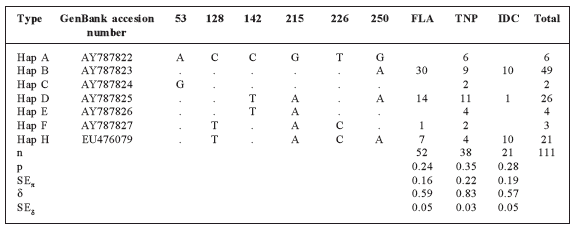
The TNP subpopulation shows the greatest haplotype diversity (δ = 0.83), with all seven haplotypes (A, B, C, D, E, F and H) present. Haplotype diversity was smaller (δ = 0.57-0.59) in the other subpopulations (Table 1). Nucleotide diversity was also higher in TNP (π = 0.35) than in IDC. Haplotype B had the highest frequency, averaged over the ATDF ![]() ; haplotype D had an average frequency of
; haplotype D had an average frequency of ![]() ; and haplotype H had an average frequency of
; and haplotype H had an average frequency of ![]() . All subpopulations shared haplotypes B, D and H, and two of the three shared haplotype F, but haplotypes A, C and E were restricted to TNP (Table 1; Fig. 2).
. All subpopulations shared haplotypes B, D and H, and two of the three shared haplotype F, but haplotypes A, C and E were restricted to TNP (Table 1; Fig. 2).

Fig. 2. Haplotype network of Castor canadensis. Areas are proportional to haplotype frequencies and shading indicates localities. Haplotype letters and localities names correspond to those shown in Table 1.
Patterns of Genetic Diversity
Our AMOVA partition of mitochondrial variation among the 111 ATDF samples (three subpopulations) yielded Fst=0.104 (p < 0.001); that is to say ~11% of the total variation was attributable to population divergence, with 89% of the variation found within subpopulations. There has been non-trivial genetic divergence since initial occupation in 1946; diversification over space has accompanied demographic growth and territorial expansion across the ATDF, a predictable outcome of the early colonization of new habitat by a small founder population. We had limited statistical power, of course, because there were only three pairwise comparisons, but all of them were significant, Fst(TNP vs. FLA) = 0.063 (p = 0.018), Fst(TNP vs. IDC) = 0.166 (p = 0.018) and Fst (FLA vs. IDC) = 0.124 (p d ± 0.001).
Pattern Analysis
A spatial autocorrelation analysis (SAA) of individual genotypes (Smouse and Peakall, 1999) was carried out for the TNP subpopulation. Even with n = 38, the autocorrelogram is estimated with considerable sampling noise, and shows oscillation of positive and negative autocorrelations. The r-values are positive and significant out to 800 m (r = 0.193, p = 0.003) and at 8 km (r = 0.120, p = 0.005), showing that genetic affinity is not spatially random.
DISCUSSION
Population structure
The settlement of a restricted number of founders in new areas often yields a founder effect, reducing genetic variation within the founding population and increasing genetic differentiation between it and the original population in absence of gene flow (Wang et al., 2008). Although we still have no pre-colonization estimate of population structure of beavers from the native range in North America for comparison, it is noteworthy that there is substantial variation within individual subpopulations (89%) and small (though significant) differentiation among them (11%) in the ATDF. Lizarralde et al. (2008a) pointed out that a maximum of 25 mitochondrial lineages could have been the founders of the ATDF invasive population, from which only seven lineages are reported here. Of course, there may not have been 25 distinct lineages represented among the founders, but it is also possible that some lineages, initially present, have subsequently become extinct, due to stochastic loss or selection, during the process of colonization and expansion.
Using an island model, Wright (1978) suggested that values of Fst = 0.25 would indicate great differentiation among subpopulations and the range 0.15-0.25 indicates moderate differentiation. In practice, Fst values are rarely larger than 0.5 and are often very much less. The complex network of aquatic habitats throughout the ATDF predicts that beavers might be moving freely throughout the region as one large panmictic population. However, our results indicate low but significant divergence among subpopulations (Fst = 0.104, p <0.001). The manner in which animals use a landscape is determined both by their habitat requirements and social structure; thus, populations may exhibit spatial genetic structure even in the absence of physical barriers (Latch et al., 2008). In this work, we found significant divergence between Isla Dawson and the subpopulations of the Isla Grande (FLA and TNP; Fst = 0.124-0.166), suggesting a substantial geographic barrier to gene flow (perhaps coupled with a founder effect), presumably represented by the oceanic channel between Isla Grande and Isla Dawson (Hoffmann, 1985). On the other hand, the moderate Fst value between the subpopulations of the Isla Grande (TNP vs. FLA, Fst = 0.063) could be due to dispersal capabilities and large distances between the subpopulations (ca. 100 km).
Spatial Genetic Structure Within TNP
In TNP, autocorrelation was positive and significant (p < 0.05) for the first distance class (0-800 m), and the fifth (5-8 km) but for the other distance class, genetic affinity fluctuates around zero and is not reliably related to distance. It is clear, however, that proximal pairs are (on average) more related than are spatially more distant pairs, a typical signature of restricted dispersal and territoriality. The results of this research are congruent with our earlier studies, showing that the beaver population in the TNP was genetically structured (Lizarralde et al., 2008a). This particular spatial genetic structure might be linked, first, to the territorial organization of beavers (800 m) and second to the migration of dispersers (subadults) constrained to travel far from the home-range of adults (5-8 km) to establish new colonies. Beavers become mature later than other rodents (2-3 years old), so subadults disperse and must settle far away to avoid costly aggressive interactions (Fustec et al. 2001). For example, European beaver might require a home-range averaging 7.9 km (Nolet and Rosell, 1994), while in C. canadensis distance travelled along the stream from 8 to 16 km (Leege 1968).
The elevated variability within the TNP population can probably be explained by (1) the greater habitat diversities in the TNP or (2) the control management used in the National Park over the last several years. Control of beaver in the National Park began in 2001 with the objective of diminishing the beaver population expansion through selective extraction of beavers. Extirpation of beavers from particular areas and destruction of dams would produce 'open patches' that could be colonized by individuals from neighboring areas, creating a highly patchy pattern of recolonization and genetic variability.
Management implications
The effectiveness of control may depend on identification of possible control points from their spatial genetic structure (labeled "eradication units" by Abdelkrim et al., 2005) i.e. evidences of migration between subpopulations can be revealed by studying genetic structure. Studies to detect possible invasion paths of the species towards continental Chile and Patagonia will be carried out using microsatellites to analyze key populations going through the invasion process. Developing control strategies based on molecular data turns out to be not only efficient in the long term, but also creative, due to spatial genetic structure characteristics. A management priority is thus to first eradicate beavers from the newly created contact zone (Peninsula Brunswick, Isla Dawson, Chile) so as to break down the connectivity between potential beaver management units separated by big rivers or oceanic channel and to prevent re-invasion of this area. Concentration of eradication effort in such subpopulations may reduce the colonization of the Continental Patagonia. Our findings of genetic structuring of invasive species and future studies in landscape features restricting gene flow can help to guide eradication and management efforts to slow down the invasion rate.
ACKNOWLEDGEMENTS
We thank Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and Federación Argentina de Comercialización e Industrialización de la Fauna (FACIF) for financial support of MSL. We also thank Julio Escobar and Guillermo Deferrari for their contributions of samples, the staff at TNP, the hunter-trappers and field collectors, and especially to Nicolás Soto (SAG, Chile) who provides us with beaver's samples from Isla Dawson (Chile) and also Julio Escobar for his invaluable knowledge of the ATDF beaver population. We are also grateful to all members of Centro Regional de Estudios Genómicos (CREG) for their ongoing support and advice. MF would like to thank Peter Smouse and Eva Gonzales for coaching on the spatial autocorrelation analyses, and especially Peter Smouse for comments on the manuscript. We offer many thanks to Matías Mora for his invaluable help and comments on the manuscript.
LITERATURE CITED
1. ABDELKRIM J, M PASCAL, C CALMET, and S SAMADI. 2005. Importance of assessing population genetic structure before eradication of invasive species: examples from insular Norway Rat populations. Conservation Biology 19:1509-1518. [ Links ]
2. ANDERSON CB, CR GRIFFITH, AD ROSEMOND, R ROSSI, and O DOLLENZ. 2006. The effects of invasive North American beavers on riparian vegetation communities in Cape Horn, Chile: do exotic beavers engineer differently in sub-Antartic ecosystems? Biological Conservation 128:467-474. [ Links ]
3. ANDERSON CB, GM PASTUR, MV LENCINAS, PK WALLEM, MC MOORMAN, and AD ROSEMOND. 2009. Do introduced North American beavers Castor canadensis engineer differently in southern South America? An overview with implications for restoration. Mammal Review 39:33-52. [ Links ]
4. AVISE JC. 1998. The history and purview of phylogeography: a personal reflection. Molecular Ecology 19:467-476. [ Links ]
5. BAKER AM, PB MATHER, and JM HUGHES. 2000. Population genetic structure of Australian magpies: evidence for regional differences in juvenile dispersal behavior. Heredity 85:167-176. [ Links ]
6. BONINO NA. 1995. Introduced mammals in Patagonia, Southern Argentina: consequences, problems, and management considerations. International Wildlife Management Congress. Pp 406-409, in: Integrating people and wildlife for a sustainable future. Proceedings of the first International Wildlife Management Congress (JA Bissonette and PR Krausman, eds.). The Wildlife Society, Bethesda. [ Links ]
7. BROWN GG, G GADALETA, G PEPE, C SACCONE, and E SBISÀ. 1986. Structural conservation and variation in the D-loop containing region of vertebrate mitochondrial DNA. Journal of Molecular Biology 192:503-511. [ Links ]
8. BRYAN MB, D ZALINSKI, KB FILCEK, S LIBANTS, W LI, and KT SCRIBNER. 2005. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by microsatellite genotypes. Molecular Ecology 14:3757-3773. [ Links ]
9. CLOUT MN and CR VEITCH. 2002. Turning the tide of biological invasion: the potential for eradicating invasive species. Pp 1-3, in: Turning the tide: the eradication of invasive species. (CR Veitch and MN Clout, eds.). World Conservation Union Species Survival Commission, Invasive Species Specialist Group, Gland, Switzerland and Cambridge, United Kingdom. [ Links ]
10. CORONATO A, J ESCOBAR, C MALLEA, C ROIG, and M LIZARRALDE. 2003. Características geomorfológicas de ríos de montaña colonizados por Castor canadensis en Tierra del Fuego, Argentina. Ecología Austral 13:15-26. [ Links ]
11. EPPERSON BK. 1990. Spatial autocorrelation of genotypes under directional selection. Genetics 124:757-771. [ Links ]
12. EXCOFFIER L, PE SMOUSE, and JM QUATTRO. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics 131:479-491. [ Links ]
13. EXCOFFIER L, G LAVAL, and S SCHNEIDER. 2005. ARLEQUIN Version 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1:47-50. [ Links ]
14. FOERSTER K, M VALCU, A JOHNSEN, and B KEMPENAERS. 2006. A spatial genetic structure and effects of relatedness on mate choice in a wild bird population. Molecular Ecology 15:4555-4567. [ Links ]
15. FRANKHAM R. 2005. Resolving the genetic paradox in invasive species. Heredity 94:385. [ Links ]
16. FUSTEC J, T LODE, D LE JACQUES, and JP CORMIER. 2001. Colonization, riparian habitat selection and home range size in a reintroduced population of European beavers in the Loire. Freshwater Biology 46:1361-1371. [ Links ]
17. GODOY JC. 1963. Inventario de la fauna exótica existente en la Argentina. Pp. 61-64, in: Fauna Silvestre, Evaluación de los Recursos Naturales, Consejo Federal de Inversiones, Buenos Aires. [ Links ]
18. HAMPTON JO, PBS SPENCER, DL ALPERS, LE TWIGG, AP WOOLNOUGH, J DOUST, T HIGGS, and J PLUSKE. 2004. Molecular techniques, wildlife management and the importance of genetic population structure and dispersal: a case study with feral pigs. Journal of Applied Ecology 41:735-743. [ Links ]
19. HARDY OJ and X VEKEMANS. 1999. Isolation by distance in a continuous population: reconciliation between spatial autocorrelation analysis and population genetics models. Heredity 83:145-154. [ Links ]
20. HOFFMANN M. 1985. Zur Ansiedlung der Bisamratte, Ondatra zibethica (L.) in Südamerika (On the settlement of the muskrat, Ondatra zibethica (L.) in South-America). Journal of Pest Science 58:93-97. [ Links ]
21. JAKSIC FM and ER FUENTES. 1980. Why are native herbs in the Chilean matorral more abundant beneath bushes: microclimate or grazing? Journal of Ecology 68:665-669. [ Links ]
22. JAKSIC FM, JA IRIARTE, JE JIMÉNEZ, and DR MARTÍNEZ. 2002. Invaders without frontiers: crossborder invasions of exotic mammals. Biological Invasions 4:157-173. [ Links ]
23. KOLAR CS and DM LODGE. 2001. Progress in invasion biology: predicting invaders. Trends in Ecology & Evolution 16:199-204. [ Links ]
24. KOLBE JA, RE GLOR, L RODRÍGUEZ SCHETTINO, A CHAMIZO LARA, A LARSON, and JB LOSOS. 2004. Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177-181. [ Links ]
25. LATCH EK, DG SCOGNAMILLO, JA FIKE, MB CHAMBERLAIN, and OE RHODES. 2008. Deciphering ecological barriers to North American River Otter (Lontra canadensis) gene flow in the Louisiana landscape. Journal of Heredity 99:265-274. [ Links ]
26. LECIS R, A FERRANDO, J RUIZ-OLMO, S MAÑAS, and X DOMINGO-ROURA. 2008. Population genetic structure and distribution of introduced American mink (Mustela vison) in Spain, based on microsatellite variation. Conservation Genetics 9:1149-1161. [ Links ]
27. LEEGE TA. 1968. Natural movements of Beaver in southeastern Idaho. Journal of Wildlife Management 32:973-976. [ Links ]
28. LINDHOLM AK, F BREDEN, HJ ALEXANDER, WK CHAN, SG THAKURTA, and R BROOKS. 2005. Invasion success and genetic diversity of introduced populations of guppies Poecilia reticulate in Australia. Molecular Ecology 14:3671-3682. [ Links ]
29. LIZARRALDE M. 1993. Current status of the introduced beaver (Castor canadensis) population in Tierra del Fuego, Argentina. Ambio 22:351-358. [ Links ]
30. LIZARRALDE MS, GA DEFERRARI, SE ALVAREZ, and JM ESCOBAR. 1996. Effects of beaver (Castor canadensis) on the nutrient dynamics of the Southern Beech forest of Tierra del Fuego (Argentina). Ecología Austral 6:101-105. [ Links ]
31. LIZARRALDE M and JM ESCOBAR. 2000. Mamíferos exóticos en la Tierra del Fuego. Ciencia Hoy 10:52-63. [ Links ]
32. LIZARRALDE M and M ELISETCH. 2002. Economic significance and standard development of mammal trapping in Argentina. Pp. 340-342, in: Wildlife, land and people: priorities for the 21st century (R Field, R Warren, and H Okarma, eds.) Wildlife Society, USA. [ Links ]
33. LIZARRALDE M and C VENEGAS. 2002. El castor un ingeniero exótico en las tierras más australes del planeta. Pp. 231-232, in: Fundamentos de Conservación Biológica: Perspectivas Latinoamericanas. (R Primack, R Rozzi, P Feisinger, R Dirzo, and F Massardo). Fondo de Cultura Económica, México. [ Links ]
34. LIZARRALDE M, J ESCOBAR, and G DEFERRARI. 2004. Invader species in Argentina: A review about the beaver (Castor canadensis) population situation on Tierra del Fuego ecosystem. Interciencia 29:352-356. [ Links ]
35. LIZARRALDE MS, G BAILLIET, S POLJAK, M FASANELLA, and C GIULIVI. 2008a. Assessing genetic variation and population structure of invasive North American beaver (Castor canadensis Kuhl, 1820) in Tierra del Fuego (Argentina). Biological Invasions 10:673-683. [ Links ]
36. LIZARRALDE M, J ESCOBAR, G DEFERRARI, and M FASANELLA. 2008b. El castor austral. Investigación y Ciencia 379:58-64. [ Links ]
37. MARTÍNEZ PASTUR G, MV LENCINAS, J ESCOBAR, P QUIROGA, L MALMIERCA, and M LIZARRALDE. 2006. Understory succession in areas of Nothofagus forests affected by Castor canadensis in Tierra del Fuego (Argentina). Applied Vegetation Science 9:143-154. [ Links ]
38. MCEACHERN MB, JM EADIE, and DH VAN VUREN. 2007. Local genetic structure and relatedness in a solitary mammal, Neotoma fuscipes. Behaviour Ecology Sociobiology 61:1459-1469. [ Links ]
39. MORA MS, EP LESSA, AP CUTRERA, MJ KITTLEIN, and AI VASALLO. 2007. Phylogeographical structure in the subterranean tuco-tuco Ctenomys talarum (Rodentia: Ctenomyidae): contrasting the demographic consequences of regional and habitat-specific histories. Molecular Ecology 16:3453-3465. [ Links ]
40. NOLET BA and F ROSELL. 1994. Territoriality and time budgets in Beaver during sequential settlement. Canadian Journal of Zoology 72:1227-1237. [ Links ]
41. NUSSEY DH, DW COLTMAN, T COULSON, LEB KRUUK, A DONALD, SJ MORRIS, TH CLUTTON-BROCK, and J PEMBERTON. 2005. Rapidly declining fine-scale spatial genetic structure in female red deer. Molecular Ecology 14:3395-3405. [ Links ]
42. NOVILLO A and RA OJEDA. 2008. The exotic mammals of Argentina. Biological Invasions 10:1333-1344. [ Links ]
43. PEAKALL R, M RUIBAL, and DB LINDENMAYER. 2003. Spatial autocorrelation analysis offers new insights into gene flow in the Australian Bush Rat, Rattus fuscipes. Evolution 57:1182-1195. [ Links ]
44. PEAKALL R and PE SMOUSE. 2006. GENAIEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6:288-295. [ Links ]
45. POLJAK S, J ESCOBAR, G DEFERRARI, and M LIZARRALDE. 2007. Un nuevo mamífero introducido en la Tierra del Fuego: el "peludo" Chaetophractus villosus (Mammalia, Dasypodidae) en Isla Grande. Revista Chilena de Historia Natural 80:285-294. [ Links ]
46. ROUSSET F. 2000. Genetic differentiation between individuals. Journal of Evolutionary Biology 13:58-62. [ Links ]
47. SACCONE C, C GISSI, C LANAVE, A LARIZZA, G PESOLE, and A REYES. 2000. Evolution of the mitocondrial genetic system: an overview. Gene 261:153-159. [ Links ]
48. SAKAI AK, FW ALLENDORF, JS HOLT, DM LODGE, and KAJ MOLOFSKY. 2001. The population biology of invasive species. Annual Review of Ecology, Evolution and Systematics 32:305-332. [ Links ]
49. SALA OE, FS CHAPIN, JJ ARMESTO, E BERLOW, J BLOOMFIELD, R DIRZO, E HUBERSANNWALD, LF HUENNEKE, RB JACKSON, A KINZIG, R LEEMANS, DM LODGE, HA MOONEY, M OESTERHELD, N LEROY POFF, MT SYKES, BH WALKER, M WALKER, and DH WALL. 2000. Global biodiversity scenarios for the year 2100. Science 287:1770-1774. [ Links ]
50. SAMBROOK J, EF FRITSCH, and T.MANIATIS. 1989. Molecular cloning: a Laboratory Manual. 2nd Edition. Cold Spring Harbor Laboratory Press, New York. [ Links ]
51. SKEWES O, F GONZÁLEZ, R OLAVE, A AVILA, V VARGAS, P PAULSEN, and H KONIG. 2006. Abundance and distribution of American Beaver, Castor canadensis (Kuhl 1820), in Tierra del Fuego and Navarino islands, Chile. European Journal of Wildlife Research 52:292-296. [ Links ]
52. SMOUSE PE and R PEAKALL. 1999. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561-573. [ Links ]
53. SMOUSE PE, R PEAKALL, and E GONZALEZ. 2008. A heterogeneity test for fine-scale genetic structure. Molecular Ecology 17:3389-3400. [ Links ]
54. TEMPLE HJ, JI HOFFMAN, and W AMOS. 2006. Dispersal, philopatry and intergroup relatedness: finescale genetic structure in the white-breasted thrasher, Ramphocinclus brachyurus. Molecular Ecology 15:3449-3458. [ Links ]
55. THOMPSON JD, DG HIGGINS, and TJ GIBSON. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research 22:4673-4680. [ Links ]
56. TRAVIS JM and KJ PARK. 2004. Spatial structure and the control of invasive alien species. Animal Conservation 7:321-330. [ Links ]
57. VALBUENA-CARABAÑA M, SC GONZÁLEZMARTÍNEZ, OJ HARDY, and L GIL. 2007. Finescale spatial genetic structure in mixed oak stands with different levels of hybridization. Molecular Ecology 16:1207-1219. [ Links ]
58. VÁZQUEZ DP. 2002. Multiple effects of introduced mammalian herbivores in a temperate forest. Biological Invasions 4:175-191. [ Links ]
59. VEKEMANS X and OJ HARDY. 2004. New insights from spatial genetic structure analyses in plant populations. Molecular Ecology 13:931-935. [ Links ]
60. VILÀ C, IR AMORIM, JA LEONARD, D POSADAS, J CASTROVIEJO, F PETRUCCI-FONSECA, KA CRANDALL, H ELLEGREN, and RK WAYNE. 1999. Mitochondrial DNA phylogeography and population history of the grey wolf Canis lupus. Molecular Ecology 8:2089-2103. [ Links ]
61. WANG T, Y SU, and G CHEN. 2008. Population genetic variation and structure of the invasive weed Mikania micrantha in Southern China: consequences of rapid range expansion. Journal of Heredity 99:22-33. [ Links ]
62. WEIR BS and CC COCKERHAM. 1984. Estimating FStatistics for the analysis of population structure. Evolution 38:1358-1370. [ Links ]
63. WRIGHT S. 1943. Isolation by distance. Genetics 28:114-128. [ Links ]
64. WRIGHT S. 1978. Evolution and the Genetics of Populations. Vol 4, in: Variability Within and Among Natural Populations. University of Chicago Press, Chicago. [ Links ]
Recibido 30 junio 2009.
Aceptado 28 octubre 2009.
Editor asociado: E Lessa














