Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Odontológica Latinoamericana
On-line version ISSN 1852-4834
Acta odontol. latinoam. vol.31 no.3 Buenos Aires Dec. 2018
ORIGINAL ARTICLE
Study of epithelial rests of Malassez in an experimental periodontitis model
Estudio de los restos epiteliales de Malassez un modelo de periodontitis experimental
Gisela E. Pulitano Manisagian1, Daniel Benedí1, Juan A. Goya1, Patricia M. Mandalunis1
1 Universidad de Buenos Aires, Facultad de Odontología, Cátedra de Histología y Embriología, Buenos Aires, Argentina,
ABSTRACT
The aim of this study was to evaluate the morphological alterations of epithelial cell rests of Malassez (ERMs) and their relationship with root resorption, in an experimental periodontitis (EP) model at 4 and 11 days. EP was induced in 14 male Wistar rats by placing a cotton thread ligature around the neck of the first lower right molar, for 4 (n=7) and 11 (n=7) days. The contralateral molar (left) was used as control. Following euthanasia, jaws were extracted and processed histologically to provide mesio-distal sections which were subject to H&E stain and histochemical detection technique with tartrate-resistant acid phosphatase (TRAP). The following histomorphometricparameters were evaluated on micrographs: bone area (BAr./TAr)(%), number of ERMs/mm2, number of cells/ERM, ERMs area (µm2), and percentage of root resorption surfaces (%RR). The results were analyzed statistically by ANOVA and Bonferronipost hoc (p≤ 0.05). Significant bone loss was observed in molars with EP compared to their controls. In the EP 4-Day group, no change was observed in the parameters with relation to the ERMs; however, in the EP 11-Day group, there was significant root resorption (%RR) (C: 3.21±3.07, EP-4D: 3.91±3.17, EP-11D: 23.67± 11.40; p≤ 0,05) and increase in ERMs area (µm2) (C: 455.87±145.42, EP-4D: 577.6±156.1, EP-11D: 1046.3± 582.9; p≤ 0,05). No TRAP+ ERM was found in either group. ERM hypertrophy may be related to ERMpartici-pation in mechanisms tending to establish periodontal homeostasis, inhibiting resorption and contributing toperiodon-tal regeneration.
Key words: Periodontitis; Root resorption; Periodontal ligament; Alveolar bone loss; Epithelial rests of Malassez.
RESUMEN
El objetivo de este trabajo ha sido evaluar las alteraciones morfológicas de epithelial cell rests of Malassez (ERMs) y su relación con la reabsorción radicular, en un modelo de experimental periodontitis (EP) a 4 y 11 días. La EP fue inducida en 14 ratas Wistar macho mediante la colocación de una ligadura de hilo de algodón alrededor del cuello del primer molar inferior derecho, a 4 (n=7) y 11 (n=7) días. El molar contralateral (izquierdo) fue usado como control. Tras la eutanasia, se extrajeron los maxilares y se procesaron histológicamente para la obtención de cortes en sentido mesio-distal que se colorearon con H&E y técnica histoquímica de detección de tartrate-resistant acid phosphatase (TRAP). Se tomaron microfotografías y se evaluaron los siguientes parámetros histomorfométricos: Bone area (BAr./TAr)(%), N° de ERMs/mm2, N° de células/ERM, área de ERMs (µm2), y porcentaje de superficies de reabsorción radicular (%RR). Los resultados se analizaron estadísticamente mediante Anova y Bonferroni post hoc (p≤ 0.05). En los molares con PE se observó una pérdida ósea significativa en relación a sus controles. En el grupo EP 4 días no se observaron cambios en los parámetros en relación a los ERMs, sin embargo, en el grupo PE de 11 días se registró reabsorción radicular (%RR) significativa (C: 3.21±3.07, EP-4D: 3.91±3.17, EP-11D: 23.67±11.40; p≤ 0,05) junto con un aumento del área de ERMs (µm2) (C: 455.87±145.42, EP-4D: 577.6±156.1, EP-11D: 1046.3±582.9; p≤ 0,05). No se observaron ERMs TRAP+ en ninguno de los dos grupos. La hipertrofia de los ERMs, podría estar relacionada a la participación de los mismos en mecanismos tendientes a la homeostasis periodontal, inhibiendo dicha reabsorción y contribuyendo a la regeneración periodontal.
Palabras clave: Periodontitis; Reabsorción radicular; Ligamento periodontal; Pérdida ósea alveolar; Restos epiteliares de Malassez.
INTRODUCTION
The insertion periodontium consists of three highly specialized connective tissues: periodontal ligament, root cementum and alveolar bone. These three structures constitute a topographic and functional unit that keeps the teeth in their respective jaws and buffers the mechanical forces exerted on them. The periodontal ligament is a fibrous connective tissue with a large number and variety of cells, including fibroblasts (main cells responsible for remodeling the ligament), osteoblasts, cementoblasts, osteoclasts, macrophages, mastocytes, epithelial cell rests of Malassez (ERMs) and undifferentiated ectomesenchymal stem cells. Of all the above, fibroblasts and perivascular ectomesenchymal stem cells play an important part during the development and homeostasis of periodontal tissues. Several signaling factors modulate the activity of these cells, which provide the machinery for tissue growth and regeneration1. The fibrillar organic component consists of two types of fibers: collagenous fibers, mainly type I, inserted in the cementum and the alveolar bone, and collagen types III and XII and elastic fibers, which include three types: elastin, oxytalan and elaunin2.
Within the study of periodontal components, it is worth highlighting the presence of the epithelial rests of Malassez (ERMs), which are groups of cells that appear during the formation of root hard tissues when the Hertwig epithelial root sheath breaks down. They persist in the periodontal ligament space throughout the lifetime of the tooth. They are unique in that they are the only odontogenic cells of epithelial nature within the periodontal structure. Studies on human ERMs3 have reported their characteristics: an irregular nucleus with dense heterochromatin and a halo of peripheral cytoplasm, small and scarcely distinguishable, and a high nucleus/cytoplasm ratio. A previous report4 describes that in rat molars, ERMs undergo increase in size, signs of apoptosis and cell proliferation with age. The authors conclude that ERMs are maintained in the periodontal ligament by cell turnover throughout the lifetime of the tooth.
Regarding ultrastructure, different studies have shown that a basal membrane separates the islands of ERMs from the connective tissue5. The presence of tonofilaments, desmosomes and hemidesmosomes has also been demonstrated, all of which anchor ERMS in the basal membrane6,7. These characteristics provide evidence of the epithelial nature of these cells.
Rincon et al.8 reported the average distance from the cementum to the ERMs in three regions: apical 21 microns, middle radicular: 33 microns, and cervical: 41 microns, indicating a coronal migration of ERMs away from the root surface towards coronal in human teeth.
ERMs have been shown to express different types of proteins and macromolecules, including cytokeratins9,10 and neuropeptides11,12,13. With regard to the expression of cytokeratins, CK 17 could be a marker to identify them14. Other studies15,16 also report the expression of cell surface proteins, including epidermal growth factor receptors. Despite their ectodermal origin, ERMs can synthesize components frequently associated to cells of mesenchymal origin, such as glycosaminoglycans, hyaluronic acid, dermatan sulfate and chondroitin sulfate17, as well as osteopontin (OPN), bone sialoprotein (BSP) and osteoprotegerin (OPG)8,18, and can also degrade collagen by synthesis of collagenases and proteinases19,20,21. It has therefore been suggested that they may make a considerable contribution to periodontal regeneration by synthesizing a series of proteins related to bone and root cementum22. The importance of ERMs in the etiopathology of odontogenic cysts and tumors is well known. Yet there is still much to learn regarding their role in periodontal diseases, especially periodontitis, which is the most frequent oral inflammatory pathology.23,24 Current scientific evidence suggests that the possible roles of ERMs in the adult periodontal ligament include maintenance of homeostasis of the periodontal medium, thereby preventing ankylosis; maintenance of the periodontal ligament space, thereby inhibiting root resorption; and contributing to repairing periodontal cementum25, 26.
The aim of this study was to conduct morphological evaluation of the epithelial rests of Malassez and to evaluate their relationship with root resorption in an experimental periodontitis model.
MATERIALS AND METHODS
The experimental protocol was approved by the Ethics Committee of the School of Dentistry 012/2016 CICUAL-ODONTO-FOUBA of Buenos Aires Argentina, and is in keeping with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Periodontitis
Experimental periodontitis (EP) was induced in 14 male Wistar rats with body weight 280 g. On Day 1, all animals were anesthetized by intraperitoneal administration of 5% Ketamine (50 mg/Kg body weight) and 2% xylazine (5mg/kg body weight). Experimental periodontitis was induced by placing a cotton thread ligature around the cervical region of the first lower right molar27 (Fig. 1). Two experimental times were used: 4 days (n=7) and 11 days (n=7). In both groups, the contralateral molar was used as a control, thereby determining 3 groups: Control Group, EP Day 4 Group and EP Day 11 Group. The animals were euthanized by intraperitoneal injection of sodium thiopental solution (150 mg/kg body weight) (Pentovent, 49 Laboratorios Richmond, Buenos Aires, Argentina) and acepromazine maleate (3 mg/kg body weight) (Acedan, Holliday-Scott S.A., Buenos Aires, Argentina).
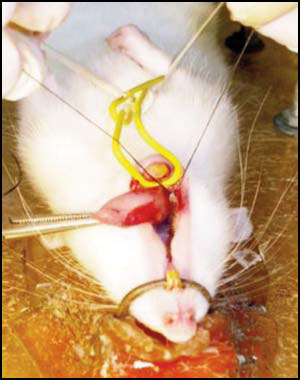
Fig. 1: Placing cotton thread ligature on first lower molar to induce experimental periodontitis (EP).
Histology and Histomorphometry
The section of each hemimandible corresponding to the three lower molars was fixed 10% buffered formalin (pH 7.4), decalcified in 10% EDTA solution, pH 7.0 for 25 days, and embedded in paraffin to prepare mesiodistally oriented histological sections of the first lower molar. The sections were a) stained with Hematoxylin-Eosin and b) subjected to histochemical determination with tartrate-resistant acid phosphatase (TRAP) to evaluate whether this enzyme, which is characteristic of cells that resorb mineralized tissues, is expressed in ERMs.
The following histomorphometric parameters were evaluated:
. Using 40x digital micrographs and Image Pro Plus software (Fig. 2):
- Bone Area (BAr/TAr)(%): Percentage of total interradicular area occupied by bone tissue.
- ERMs area (µm2) of the ERMS in the interradicular space.
- Root resorption (% RR): Percentage of surfaces in resorption, with or without presence of odontoclasts on the root surface.
. Using direct 100x bright field microscopy:
- ERMs number/mm2: count of the number of ERMs in the interradicular space (Fig. 2).
- Cell number /ERM: count of cell nuclei observable in each ERM.
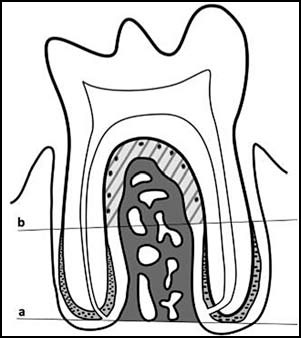
Fig. 2: Diagram of rat molar in situ. Above line a is the area used to calculate bone area (BAr/TAr)(%):. Above line b, marked at the beginning of acellular cementum, the periodontal area is shown (shaded and hatched) used for conducting ERM histomorphometric measurements.
comparison to the contralateral molar (Fig. 3, Table 1). In 4-day experimental periodontitis, no statistically significant change was observed in the other study parameters. However, at 11 days of periodontal disease, there was a statistically significant increase in root resorption (%RR) and ERMs area (Fig.4). Table 2 shows the values recorded for histomorpho-metric measurements. No TRAP-positive ERM was found in either of the study groups.
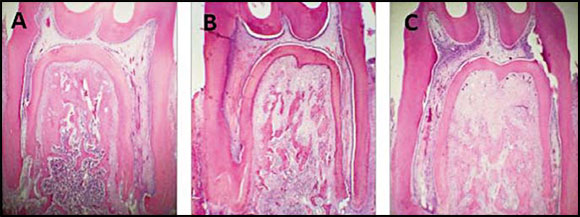
Fig. 3: A, B, C: Bright field micrographs. Decalcification technique. H&E Stain. A Lower molar in situ corresponding to control group; B Molar with 4day experimental periodontitis. C Molar with 11day experimental periodontitis. Note the reduction in bone area (BAr/TAr) in the groups with EP. 40X.
Table 1.
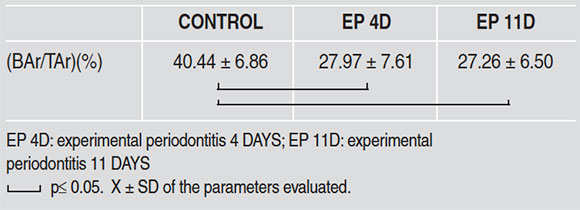
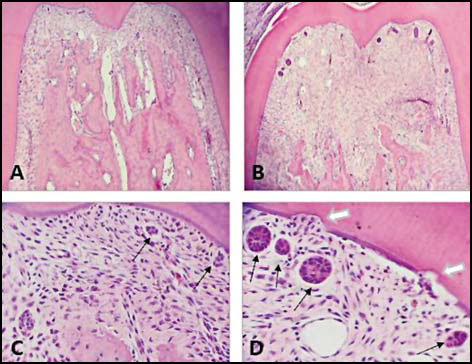
Fig. 4: Bright field micrographs. Decalcification technique. H&E Stain. Root furcation zone of first lower molar. A. Control; B. Molar with 11day experimental periodontitis. Note bone loss and ERMs. C. Control: ERMs visible (black arrows). D Molar with 11day experimental periodontitis. Note enlarged ERMs (black arrows) and root resorption areas (white arrows). A and B: 100 X; C and D: 400 X.
Table 2.
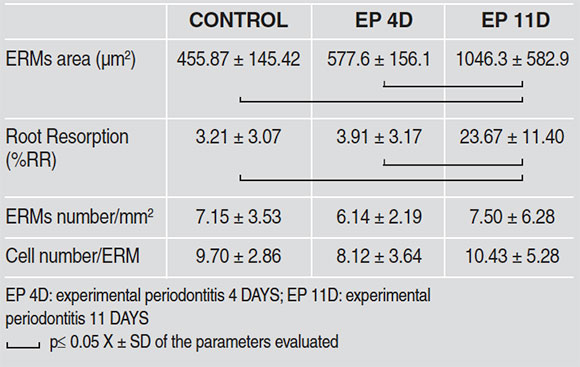
Results were analyzed statistically using ANOVA and Bonferroni post hoc, considering p≤ 0.05 as significant.
RESULTS
Bone loss in the interradicular space was observed at 4 and 11 days after induction of periodontitis, in
DISCUSSION
The results of this study show that at both 4 and 11 days after periodontitis was installed, there was bone loss at the level of the root furcation. In addition, at 11 days there was marked root resorption accompanied by a significant increase in the size of the ERMs. This morphological occurred at a result of an increase in volume, not a greater number of cells. The data suggest an association between the onset of root resorption and the morphological changes detected in the ERMs. To date, there is no published paper reporting ERM behavior as a result of an infectious/inflammatory stimulus such as experimental periodontitis. The question is whether ERMs are involved in the induction of root resorption or whether their persistence and hypertrophy are related to the expression of signaling factors associated to their potential participation in the regulation/inhibition of said resorption, as part of the homeostasis in the periodontal ligament.
In this regard, the current study performed histo-chemical determination of tartrate-resistant acid phosphatase (TRAP) with the aim of ascertaining whether ERMs express that enzyme, which characterizes cells that resorb mineralized tissues. These ERMs were found to be negative to histo-chemical marking, suggesting that they do not participate directly in the root resorption observed in the group with experimental periodontitis at 11 days.
Another study on rats subjected to in vivo orthodontic forces found that ERMs responded to experimental orthodontics by proliferating and increasing in size28. These findings partially agree with the current study, which found ERM hypertrophy but no increase in number. Other papers28'29,30 have reported that orthodontics can stimulate ERMs to secrete different factors, contributing to maintaining normal periodontal structure and function. Consolaro29 reported that ankylosis occurs when cementoblasts and ERMs are absent.
Studies on mechanical forces in vitro have reported that ERMs express heat shock proteins, vascular endothelial growth factor (VEGF), osteopontin (OPN)26 and HSP 7031. The expression of these proteins may contribute to the maintenance of cementogenesis and osteogenesis, and in addition, particularly HSP 70 may play a part by protecting against different factors, including oxidants, inflammation, hypoxia, hyperthermia and mechanical stimuli such as orthodontic forces32. Hasegawa et al.33 used Nakane's root resorption model (by mechanical injury) to study ERMs 7 days after injury, and observed ERMs adjacent to resorbed surfaces. Immunohistochemical tests revealed that the ERMs expressed BMP-2, osteopontin and ameloblastin, suggesting that they may participate in periodontal repair.
All of the aforesaid suggests that ERM hypertrophy in the current study at 11 days after induction of experimental periodontitis may be related to ERM participation in mechanisms that tend to maintain periodontal homeostasis.
As mentioned above, ERMs can express different proteins of epithelial nature14 and typical of the enamel matrix. Nishio et al.34 identified two novel proteins, apin (APIN) and amelotin (AMTN), produced by ameloblasts and junctional epithelium, and assessed whether ERMs express them under normal conditions and under disruption of periodontal integrity. They found that after a lesion, ERMs increased in size and they only obtained immunodetection of APIN, suggesting that its expression may lead to the activation of ERMs during periodontal healing and regeneration.
More recently, new findings suggest that within ERMs there is a cell population with stem cell-like characteristics and that in an adequate microenvironment, this cell population can differentiate into cells that produce mineralized matrices35.
Takahashi et al.36 examined the expression of amelogenin and ameloblastin, metallopeptidase (MMP 20) and kallikrein (KLK-4) in ERMs and fibroblasts in culture, from samples of human periodontal ligament. Immunohistochemical analysis revealed that those proteins were expressed weakly by ERMs and were not detected in periodontal fibroblasts. Previous studies suggest that amelogenin and ameloblastin may both have activity as growth factors, as well as participating in cell bonding, proliferation, migration and differentiation of fibroblasts of the periodontal ligamentt37,38. Given the reported capacity of amelogenin39 and its clinical use, it is important to conduct further studies on ERMs, considering their potential to induce regeneration of periodontal tissues by synthesizing proteins such as amelogenins.
CONCLUSIONS
This study found that the ERMs present in the periodontium of rat teeth with experimental periodontitis with 11 days' evolution reacted to root resorption in those teeth, with evident increase in size. Taking into account the information currently available in the literature, this behavior may be related to ERM participation in mechanisms that tend to maintain periodontal homeostasis by inhibiting the resorption process. Further studies are needed to learn about the behavior of these cell groups in response to stimuli of various origins, and their impact on periodontal regeneration.
FUNDING
This work was supported in part by a grant from the University of Buenos Aires, UBACyT Program N° 20020160100034.
CORRESPONDENCE
Dr. Gisela Pulitano Manisagian,
Cátedra de Histología y Embriología,
Facultad de Odontología,
Marcelo T. de Alvear 2142, 1°A,
(C1122AAH) CABA. Argentina
giselapulitano@gmail.com
1. Benatti BB, Silverio KG, Casati MZ, Sallum EA, Nociti NH Jr. Physiological features ofperiodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J Biosci Bioeng 2007; 103:1-6. [ Links ]
2. Nanci A, Bosshardt DD. Structure ofperiodontal tissues in health and desease. Periodontol 2000 2006; 40:11-28. [ Links ]
3. Inove T, Enokiya Y, Hashimoto S, Fukumashi K, Shimono M. Homeostatic factors in periodontal ligament after wound healing. Effects ofMalassez's epithelial rests. Jpn J Oral Biol 1993; 37:58-69. [ Links ]
4. Oka K, Morokuma M, Imanaka-Yoshida K, Sawa Y, Isokawa K, Honda MJ. Cellular turnover in epithelial rests of Malassez in the periodontal ligament of the mouse molar 2012; 120:484-494. doi: 10.1111/eos.12003 [ Links ]
5. Valderhaug JP, Nylen MU. Function of epithelial rests as suggested by their ultrastructure. J Periodont Res 1966; 1:69-78. [ Links ]
6. Beertsen W, Everts V. Autodesmosomes in epithelial cells of rests of Malassez in the incisor and molar periodontal ligament of the mouse. Arch Oral Biol 1979; 24:239-241. [ Links ]
7. Berkovitz BK, Whatling R, Barrett AW, Omar SS. The structure of bovine periodontal ligament with special reference to the epithelial cell rests. J Periodontol 1997; 68:905-913. [ Links ]
8. Rincon JC, Young WG, Bartold PM. The epithelial cell rests of Malassez-a role in periodontal regeneration? J Periodontal Res 2006; 41:245-252. [ Links ]
9. Gao Z, Mackenzie IC, Williams DM, Cruchley AT, Leigh I, Lane EB. Patterns of keratin-expression in rests of Malassez and periapical lesions. J Oral Pathol 1988; 17: 178-185. [ Links ]
10. Peters BH, Peters JM, Kuhn C, Zoller J, Franke WW. Maintenance of cell-typespecifi cytoskeletal character in epithelial cells out of epithelial context. Cytokeratins and other cytoskeletal proteins in the rest of Malassez of the periodontal ligament. Differentiation 1995; 59:113-126. [ Links ]
11. Heyeraas KJ, Kvinnsland I, Byers MR, Jacobsen EB. Nerve fibers immunoreactive to protein gene product 9.5, calcitonin gene-related peptide, substance P, and neuropeptide Y in the dental pulp, periodontal ligament, and gingiva in cats. Acta Odontol Scand 1993; 51:207-221. [ Links ]
12. Beck F, Tucci J, Russell A, Senior PV, Ferguson MW. The expression of the gene coding for parathyroid hormone-related protein (PTHrP) during tooth development in the rat. Cell Tissue Res 1995; 280:283-290. [ Links ]
13. Tadokoro O, Maeda T, Heyeraas KJ, Vandevska- Radunovic V, Kozawa Y, Hals Kvinnsland I. Merkel-like cells in Malassez epithelium in the periodontal ligament of cats: an immunohistochemical, confocal-laser scanning and immuno electron-microscopic investigation. J Periodont Res 2002; 37:456463. [ Links ]
14. Li S, Ge S, Yang P. Expression of cytokeratins in enamel organ, junctional epithelium and epithelial cell rests of Malassez. J periodontal Res 2015; 50:846-854. [ Links ]
15. Onishi T, Ooshima T, Sobue S, Tabata MJ, Maeda T, Kurisu K, Wakisaka S. Immunohistochemical localization of calbindin D28k during root formation of rat molar teeth. Cell Tissue Res 1999; 297:503-512. [ Links ]
16. Guajardo G, Okamoto Y, Gogen H, Shanfeld JL, Dobeck J, Herring AH, Davidovitch Z. Immunohistochemical localization of epidermal growth factor in cat paradental tissues during tooth movement. Am J Orthod Dentofacial Orthop 2000; 118:210-219. [ Links ]
17. Merrilees MJ, Sodek J, Aubin JE. Effects of cells of epithelial rests of Malassez and endothelial cells on synthesis of glycosaminoglycans by periodontal ligament fibroblasts in vitro. Dev Biol 1983; 97:146-153. [ Links ]
18. Rincon JC, Xiao Y, Young WG, Bartold PM. Production of osteopontin by cultured porcine epitheliacell rests of Malassez. J Periodont Res 2005; 40:417-426. [ Links ]
19. Birek P, Wang HM, Brunette DM, Melcher AH. Epithelial rests of Malassez in vitro. Phagocytosis of collagen and the possible role of their lysosomal enzymes in collagen degradation. Lab Invest 1980; 43:61-72. [ Links ]
20. Firth JD, Putnins EE, Larjava H, Uitto VJ. Bacterialmatrix metalloproteinase expression by cultured epithelial cells. Infect Immun 1997; 65:4931-4936. [ Links ]
21. Uitto VJ, Airola K, Vaalamo M, Johansson N, Putnins EE, Firth JD, Salonen J, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Collagenase-3 (matrix metalloproteinase- 13) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol 1998; 152:1489-1499. [ Links ]
22. Yang Z, Li Y, Ma X, Shen L, Zhao Z, Jin F. Role of the Epithelial Cell Rests of Malassez in Periodontal Homeostasis and Regeneration - A Review. Curr Stem Cell Res Ther 2015; 10:398-404. [ Links ]
23. Nazar Majeed Z, Philip K, Alabsi AM, Pushparajan S, Swaminathan D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis Markers 2016; 2016:1804727. doi: 10.1155/2016/1804727 [ Links ]
24. Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 2009; 36:458-467. doi: 10.1111/j.1600-051X.2009. 01408.x [ Links ]
25. Xiong J, Gronthos S, Bartold PM. Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol 2000 2013; 63:217-233. [ Links ]
26. Koshihara T, Matsuzaka K, Sato T, Inoue T. Effect of stretching Force on the Cells of Epithelial Rests of Malassez In Vitro. Int J Dent 2010; 2010:458408. doi: 10.1155/2010/458408 [ Links ]
27. Ubios AM, Costa OR, Cabrini RL. Early steps in boneresorption in experimental periodontitis: A histomorphometric study. Acta Odontol Latinoam 1993; 7:45-50. [ Links ]
28. Talic NF, Evans CA, Daniel JC, Zaki AE. Proliferation of epithelial rests of Malassez during experimental tooth movement. Am J Orthod Dentofacial Orthop 2003; 123: 527-533. [ Links ]
29. Consolaro A. The concept of root resorptions or root resorptions are not multifactorial, complex, controversial or polemical! Dental Press J Orthod 2011; 16:19-24. [ Links ]
30. Yamanaka T, Sakamoto A, Tanaka Y, Zhang Y, Hayashido Y, Toratani S, Akagawa Y, Okamoto T. Isolation and serumfree culture of epithelial cells derived from epithelial rests of Malassez in human periodontal ligament. In Vitro Cell Dev Biol Anim 2000; 36:548-553. [ Links ]
31. Kogai H , Nakajima K, Ser-Od T, Al-Wahabi A, Matsuzaka K, Nakagawa T, Inoue T. HSP70 mRNA expression by cells of the epithelial rest of Malassez due to mechanical forces in vitro. BMC Oral Health 2016; 16:22. doi: 10.1186/s 12903-016-0181-4 [ Links ]
32. Muraoka R, Nakano K, Matsuda H, Tomoda M, Okafuji N, Yamada K, Kawakami T, Kawakami T. A consideration on the role of HSP70 appearing in the periodontal tissue due to experimental orthodontic force. J Hard Tissue Biol 2011; 20:275-282. [ Links ]
33. Hasegawa N, Kawaguchi H, Ogawa T, Uchida T, Kurihara H. Immunohistochemical characteristics of epithelial cell rests of Malassez during cementum repair. J Periodont Res 2003; 38:51-56. [ Links ]
34. Nishio C, Wazen R, Kuroda S, Moffatt P, Nanci A. Disruption of periodontal integrity induces expression of apin by epithelial cell rests of Malassez. J Periodont Res 2010; 45:709-713. [ Links ]
35. Tsunematsu T, Fujiwara N, Yoshida M, Takayama Y, Kujiraoka S, Qi G, Kitagawa M, Kondo T, Yamada A, Arakaki R, Miyauchi M, Ogawa I, Abiko Y, Nikawa H, Murakami S, Takata T, Ishimaru N, Kudo Y. Human odontogenic epithelial cells derived from epithelial rests of Malassez possess stem cell properties. Lab Invest 2016; 96:1063-1075. doi: 10.1038/labinvest.2016.85 [ Links ]
36. Takahashi K, Shimonishi M, Wang R, Watanabe H, Kikuchi M. Epithelial- mesenchymal interactions induce enamel matrix proteins and proteases in the epithelial cells of the rests of Malassez in vitro. Eur J Oral Sci 2012; 120:475483. [ Links ]
37. Zeichner-David M, Chen L-S, Hsu Z, Reyna J, Caton J, Bringas P. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur J Oral Sci 2006; 114:244-253. [ Links ]
38. Kémoun P, Gronthos S, Snead ML, Rue J, Courtois B, Vaysse F, Salles JP, Brunel G. The role of cell surface markers and enamel matrix derivatives on human periodontal ligament mesenchymal progenitor responses in vitro. Biomaterials 2011; 32:7375-7388. doi: 10.1016/j.biomaterials. 2011.06.043 [ Links ]
39. Haruyama N, Hatakeyama J, Moriyama K, Kulkarni A. Amelogenins: Multi-functional enamel matrix proteins and their binding partners. J Oral Biosci 2011; 53:257266. [ Links ]














